文章信息
- 程道海,陆华,黄振光,覃兴昆.
- CHENG Daohai, LU Hua, HUANG Zhenguang, QIN Xingkun.
- 联合用药对大剂量甲氨蝶呤化疗治疗儿童急性淋巴细胞白血病肾毒性及血药浓度的影响
- Influence of Drug Combinations on High-dose Methotrexate-induced Nephrotoxicity and Blood Concentrations of Childhood Acute Lymphoblastic Leukemia
- 肿瘤防治研究, 2015, 42(11): 1148-1151
- Cancer Research on Prevention and Treatment, 2015, 42(11): 1148-1151
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2015.11.020
-
文章历史
- 收稿日期: 2014-11-17
- 修回日期: 2015-01-04
大剂量甲氨蝶呤(methotrexate, MTX)加亚叶酸钙(calcium folinate, CF)结合三联鞘注是治疗儿童急性淋巴细胞白血病(acute lymphoblastic leukemia, ALL)髓外白血病的主要手段。大剂量MTX化疗后出现急性肾损伤(acute kidney injury, AKI)是其严重并发症。在影响MTX体内蓄积及肾毒性的众多因素中,药物相互作用占重要地位,而国内有关这方面的研究较少。所以本文回顾性收集我院行大剂量MTX化疗的儿童ALL患者的临床资料,探讨联合用药对大剂量MTX化疗肾毒性及MTX血药浓度的影响,为临床防范大剂量MTX毒性反应和提高治疗安全性提供参考。
1 资料与方法 1.1 资料来源本文资料来源于广西医科大学第一附属医院儿科2012年9月至2014年9月住院的178例ALL患者,行大剂量MTX化疗共计633例次。根据儿童ALL诊疗建议[1],将患者按年龄、白细胞计数、免疫分型等因素分为低危型、中危型和高危型,于巩固治疗达到完全缓解后,血象及肝肾功能无异常时开始行大剂量MTX化疗。
1.2 化疗方法根据患者疾病类型每个疗程使用MTX剂量 3~5 g/m2,1/6量(不超过500毫克/次)作为突击量在30 min内避光快速静脉滴入,余量于23.5 h内避光均匀滴入。突击量MTX滴入后1 h,行腰椎穿刺+三联(0.9%氯化钠溶液10 ml+MTX 12.5 mg+阿糖胞苷35 mg+地塞米松5 mg)鞘内注射1次。开始滴注MTX 36 h用CF解救,按体表面积首次剂量为30 mg/m2,之后15 mg/m2,每6 h一次,其后根据MTX血药浓度监测结果进行调整[2]。均于化疗前一天开始行碱化尿液,化疗当天和用药后3天大量水化、碱化尿液[1]。在行大剂量MTX同时,每晚顿服硫鸟嘌呤片50 mg/m2,共7天。
1.3 血药浓度监测MTX血药浓度自开始滴注MTX 48 h监测,取血浆置于血药浓度测定仪(西门子公司,型号Viva-E)进行检测,以后每隔24 h监测1次,直至MTX血药浓度降至安全范围(<0.2 μmol/L)。
1.4 AKI诊断标准依据改善全球肾脏病预后组织(KDIGO)AKI指南[3],患者符合以下情况之一即可诊断AKI:(1)48 h内Scr升高超过26.5 μmol/L;(2)7天内Scr升高超过基线1.5倍;(3)尿量持续6 h以上每小时<0.5 ml/kg,并根据Scr和尿量对AKI严重程度分为轻度、中度和重度。
1.5 联合用药统计青霉素类、非甾体类抗炎药(NSAIDs)及质子泵抑制剂(PPIs)等是儿童ALL患者的常用药物,此类药物与MTX联用时易导致MTX肾毒性增加或延缓MTX的清除[4]。本研究统计在大剂量MTX化疗前一天、化疗当天和用药后3天内是否全身应用上述药物,不包括皮试和局部外用等用法。在633例次大剂量MTX治疗中,有84例次联合应用了哌拉西林、布洛芬和奥美拉唑等药物,见表 1。
采用SPSS 22.0软件分析,计数资料比较采用χ2检验,计量资料比较采用秩和检验,P<0.05为差异有统计学意义。
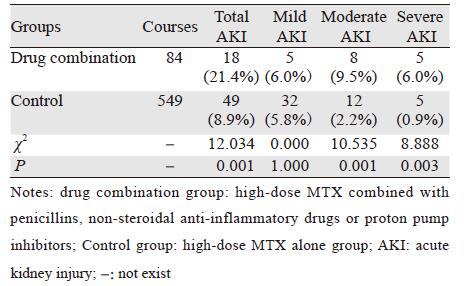
2 结果 2.1 联合用药组与对照组AKI发生率比较联合用药组AKI总体发生率明显高于对照组(P<0.05),表现在中度和重度AKI发生率明显增加(P均<0.05),见表 2。
 |
联合用药组各时间点MTX血药浓度明显高于对照组(P<0.05),MTX血药浓度降至安全范围所需天数明显长于对照组(P<0.05),见表 3。
 |
MTX主要以原型经肾脏排泄,包括肾小管分泌和肾小球滤过等过程,MTX与其他药物间的相互作用主要是发生在肾小管分泌过程[5]。青霉素类如阿莫西林、哌拉西林、氨苄西林等可竞争性抑制人有机阴离子转运蛋白(hOAT)介导的MTX转运,使MTX从肾小管分泌减少,引起MTX体内蓄积,全身毒性增加[6]。NSAIDs与MTX联用时,可通过与MTX竞争hOAT如hOAT1、hOAT3、hOAT4[5]或多药耐药蛋白(MRP)如MRP2、MRP4[7]等转运蛋白,从而减少肾小管对MTX的分泌,使MTX排泄减少。目前,MTX常与NSAIDs联用治疗自身免疫性疾病,但由于MTX口服剂量极低,因而较为安全[8]。PPIs类如奥美拉唑和泮托拉唑可能通过抑制近曲小管乳腺癌耐药蛋白(BCRP)减少MTX分泌,使血清MTX水平升高而增加毒性[9]。磺胺类药物则可能通过竞争结合血浆蛋白使游离MTX浓度增高[4]。其他可能引起MTX排泄延迟的药物还包括丙磺舒、水杨酸类、氯霉素等,但在本资料中未见有联用。
MTX肾毒性的机制主要是MTX及其代谢产物7-羟基-MTX(7-OH-MTX)沉积于肾小管,形成结晶性肾病变[10]。本研究结果,提示联用后MTX排泄延缓,肾毒性发生风险相应增加。为确保儿童ALL治疗的安全性,临床应用时应注意避免这些药物与大剂量MTX的合用,必须联用时应密切监测MTX血药浓度和肾功能变化。一旦发生AKI,需积极处理以减少严重并发症,措施包括加大CF解救剂量,加强水化、碱化和保护消化道黏膜等支持治疗,对于肾脏严重受损及MTX血药浓度明显升高者,应及早采取高通量血液透析进行治疗[11]。
本研究中,对照组的AKI发生率为8.9%,且部分MTX血药浓度高于文献报道的数值(正常排泄时48 h MTX血药浓度在0.1~1 μmol/L,96 h MTX血药浓度在0.1 μmol/L以下)[12]。说明MTX蓄积和肾毒性的发生除了与合并用药有关外,还可能与其他因素有关。有研究表明,当患者存在第三空间(如胸腔积液、腹水或手术后的伤口积液等)[13]或亚甲基四氢叶酸还原酶677CC基因表型[14]时较易发生MTX排泄延迟,肾毒性发生风险增加。因此,当存在这类危险因素时,行大剂量MTX化疗必须谨慎。
| [1] | Hematology Section of the Pediatrics Branch at the Chinese Medical Association, Editorial board of Chinese Journal of Pediatrics. Diagnosis and treatment of pediatric acute lymphoblastic leukemia (3rd revised)[J].Zhonghua Er Ke Za Zhi, 2006, 44(5): 392-5. [中华医学会儿科学分会血液学组,中华儿科杂志编辑委员会. 儿童急性淋巴细胞白血病诊疗建议(第三次修订草案)[J].中华儿科杂志, 2006, 44(5): 392-5.] |
| [2] | Ye H, Gu LJ, Chen J, et al. High dose methotrexate therapy in childhood acute lymphoblastic leukemia[J]. Zhonghua Xue Ye Xue Za Zhi, 2001, 22(7): 385-6. [叶辉, 顾龙君, 陈静, 等. 儿童急性淋巴细胞白血病大剂量甲氨蝶呤治疗研究[J]. 中华血液学杂志, 2001, 22(7): 385-6.] |
| [3] | Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury[J]. Kidney Int, 2012, 2(1 Suppl): 1-138. |
| [4] | Pang L, Liu LM, Zhao LM. Research progress in influence factors of excretion delay of high-dose methotrexate[J]. Zhongguo Yao Xue Za Zhi, 2013, 48(22): 1892-6. [庞露, 刘立民, 肇丽梅. 大剂量甲氨蝶呤排泄延迟影响因素的研究进展[J].中国药学杂志, 2013, 48(22): 1892-6.] |
| [5] | Takeda M, Khamdang S, Narikawa S, et al. Characterization of methotrexate transport and its drug interactions with human organic anion transporters[J]. J Pharmacol Exp Ther, 2002, 302(2): 666-71. |
| [6] | Zarychanski R, Wlodarczyk K, Ariano R, et al. Pharmacokinetic interaction between methotrexate and piperacillin/tazobactam resulting in prolonged toxic concentrations of methotrexate[J]. J Antimicrob Chemother, 2006, 58(1): 228-30. |
| [7] | El-Sheikh AA, van den Heuvel JJ, Koenderink JB, et al. Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport[J]. J Pharmacol Exp Ther, 2007, 320(1): 229-35. |
| [8] | Skeith KJ, Russell AS, Jamali F, et al. Lack of significant interaction between low dose methotrexate and ibuprofen or flurbiprofen in patients with arthritis[J]. J Rheumatol, 1990, 17(8): 1008-10. |
| [9] | Breedveld P, Zelcer N, Pluim D, et al. Mechanism of the pharmacokinetic interaction between methotrexate and benzimidazoles: potential role for breast cancer resistance protein in clinical drug-drug interactions[J]. Cancer Res, 2004, 64(16): 5804-11. |
| [10] | Perazella MA. Crystal-induced acute renal failure[J]. Am J Med, 1999, 106(4): 459-65. |
| [11] | Li D, Li XZ. Therapeutic effect of hemodialysis on acute kidney injury induced by high-dose methotrexate chemotherapy in children[J].Shi Yong Er Ke Lin Chuang Za Zhi, 2012, 27(17): 1320-2. [李迪, 李晓忠. 血液透析治疗大剂量甲氨蝶呤化疗所致急性肾损伤的疗效[J].实用儿科临床杂志, 2012, 27(17): 1320-2.] |
| [12] | Xu WQ, Tang YM, Fang CQ, et al. Study on elimination delay in high dose methotrexate therapy in childhood acute lymphoblastic leukemia[J].Zhonghua Xue Ye Xue Za Zhi, 2005, 26(1): 15-8. [徐卫群, 汤永民, 方澄清, 等. 大剂量甲氨蝶呤治疗儿童急性淋巴细胞白血病排泄延迟分析[J].中华血液学杂志, 2005, 26(1): 15-8.] |
| [13] | Meng LY, Tian HP, Wang XJ, et al. The effect of MTHFR gene polymorphism in the toxic side effects of HD-MTX chemotherapy on children[J].Er Ke Yao Xue Za Zhi, 2013, 19(2): 1-4. [孟琳懿, 田怀平, 王小洁, 等. 亚甲基四氢叶酸还原酶基因多态性对儿童甲氨蝶呤化疗后毒副反应[J].儿科药学杂志, 2013, 19(2): 1-4.] |
| [14] | Lu H, Zhong XB, Huang ZG, et al. Elimination delay of high-dose methotrexate in children with acute lymphoblastic leukemia[J].Zhongguo Yao Fang, 2009, 20(29): 2265-8. [陆华, 钟小斌, 黄振光, 等. 大剂量甲氨蝶呤治疗儿童急性淋巴细胞白血病排泄延迟分析[J].中国药房, 2009, 20(29): 2265-8.] |
 2015, Vol. 42
2015, Vol. 42

