文章信息
- 袁晓露, 陈琼荣. 2015.
- Xp11.2易位/TFE-3基因融合相关性肾癌1例并文献复习
- 肿瘤防治研究, 2015, 42(01): 99-100
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2015.01.025
-
文章历史
- 收稿日期:2014-04-16
- 修回日期:2014-08-15
Xp11.2易位/TFE-3基因融合相关性肾癌(renal carcinoma associated with Xp11.2 translocations/ TFE-3 gene fusions)在2004年WHO分类里首次 被归为一个亚型[1],各个年龄段(2~79岁)均可 发生,但主要见于儿童和年轻人,约占此类人群 中肾癌比例的1/3[2],而在中老年肾癌患者中仅占 1.6%(4/244)[3]。近来研究发现,Xp11.2易位/ TFE-3基因融合相关性肾癌在青幼年患者中临床生 物学行为与中老年人相比,可能有明显不同[4, 5]。 由于该肿瘤比较少见,现对我院确诊的一病例进 行复习,以加深对其认识。 1 临床资料
患者,女,20岁。以无痛性、间断性肉眼血 尿半月入院,无尿频、尿急等症状。无明显诱因 及遗传病史。入院一般体格检查:T36.8℃,R19 次/分,BP110/70 mmHg。双肾区无叩击痛。尿常 规:白细胞75(参考值0~25 μl),隐血+++,尿 蛋白+,浊度++。CT示右肾上极可见一直径3.8 cm 略高密度影的实质性占位,见图 1,临床诊断肾脏 肿瘤,全麻下行右侧肾脏肿瘤根治术。术后病理 检查结果:送检右肾脏标本,距肾上极纤维被膜 0.8 cm处肾实质中可见一直径为4 cm肿块,与周围 肾组织边界清楚,红褐色、质软。另送右肾门淋 巴结1枚,直径1.5 cm,切面灰红色质软。肿瘤细 胞排列成巢状,见图 2A、乳头状见图 2B、腺泡状 结构,见图 2C,其中以巢状结构为主,约占瘤组 织50%;乳头状结构约占30%,其内衬纤维血管轴 心;肿瘤中央约20%区域呈腺泡状结构,腺泡腔 内伴出血,形态很类似透明细胞肾细胞癌伴囊性 变。瘤细胞胞质丰富、透明或嗜酸性,核仁明显, 见图 2D,核分裂相罕见。在瘤巢的周边可见较 多的砂砾体,见图 2A,肿瘤中央有片状坏死和出 血。肾门淋巴结1枚可见皮质内有小团瘤组织转移 灶。免疫组织化学结果:TFE-3瘤细胞核强阳性, 见图 3,Ki67阳性率小于1%,其他指标(CD10、 广谱CK、CK7、CK8/18、EMA、Vimentin、 HMB45、S-100、CgA、Syn)均阴性。病理诊断: (右肾)Xp11.2易位/TFE-3基因融合相关性肾癌伴 有同侧肾门淋巴结转移1枚,肾纤维被膜及肾周脂 肪囊未受侵犯。
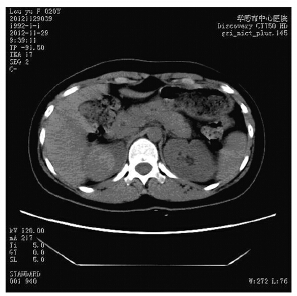
|
| 图 1 CT示Xp11.2易位/TFE-3基因融合相关性肾癌患者右 肾上极可见略高密度影实质性占位 Figure 1 One substantial occupying lesion with slightly higher-density image at the upper pole of the right kidney of renal carcinoma associated with Xp11.2 translocations/ TFE-3 gene fusions patient showed by CT scan |

|
| A: the tumor tissue was nest-shaped with marked psammoma bodies in peripheral area(HE ×40); B: tumor cells were arranged as papillary-shaped with clear to pale eosinophilic cytoplasm in some area (HE ×40); C: alveolar-shaped tumor tissues with hemorrhage were similar to cystic change of clear cell carcinoma (HE ×100); D: tumor cells showed strong atypia with prominent nucleoli(HE ×400) 图 2 Xp11.2易位/TFE-3基因融合相关性肾癌患者瘤组织HE染色结果 Figure 2 HE staining result of the tumor tissue of renal carcinoma associated with Xp11.2 translocations/TFE-3 gene fusions patient |

|
| 图 3 TFE3染色示Xp11.2易位/TFE-3基因融合相关性肾癌 患者瘤组织肿瘤细胞核强阳性(免疫组织化学染色 ×100) Figure 3 Strong positive expression of tumor cells nucleus of renal carcinoma associated with Xp11.2 translocations/TFE-3 gene fusions patient showed by TFE3 immunostaining(IHC ×100) |
Xp11.2易位/TFE-3基因融合相关性肾癌较为少 见,基因组中存在Xp11.2染色体几种不同的易位导 致TFE-3融合基因,并发挥异常转录因子的作用, 导致TFE-3融合蛋白表达升高。不仅如此,Xp11.2 易位/TFE-3基因融合相关性肾癌还在组织病理学上 有其独特特点。
病理组织学特点:透明细胞构成的乳头状结 构,常伴有由嗜酸性颗粒胞浆的瘤细胞组成巢状结 构。Rao等[6]通过对17例Xp11.2易位/TFE-3肾癌形 态学特点归纳发现47%的病例具有巢状为主型的结 构,12%的具有乳头状为主型的结构,35%的两者兼有;肿瘤细胞学特点以透明细胞为主的占53%, 以嗜酸性为主的仅为6%,两者都有的占41%。其他 特点如显著的细胞核仁、坏死、砂砾体、玻璃样变间 质、色素、胆固醇结晶、泡沫巨噬细胞依次为88%、 59%、47%、47%、29%、18%、12%。以上形态学分 析表明,无论哪类基因融合亚型都没有绝对组织结 构或细胞学特异性,因此诊断仍需要进一步免疫组 织化学和(或)FISH来确定是否有TFE3表达[7]。
本例患者年龄、组织学形态特征、TFE3免疫 组织化学检测结果都符合WHO及国外文献的诊断 标准,因此没有必要做进一步FISH检测。其他上 皮类标记如广谱CK、EMA等在Xp11.2易位/TFE-3 基因融合相关性肾癌中不表达或仅局灶表达;但 本例经多次免疫组织化学检测CD10均阴性,这与 绝大多数文献报道的CD10阳性率100%的结果不一 致[2, 4, 5, 8],而仅有少量报道CD10阴性[9],原因尚不清 楚,有待进一步的研究。
本例患者临床分期为Ⅲ期(T1aN1M0),术后 病理证实已有1枚肾门淋巴结转移。许多研究表明 该肿瘤在确诊时,近半数已有淋巴结转移[9, 10],常 见远处转移部位还包括肝脏、肺、骨、脑[5, 8, 11]。年 轻患者即使处在进展期,肿瘤仍表现出比较惰性 的过程[2, 12, 13, 14, 15],本例增殖指数低(Ki67阳性率小于 1%),与此情况符合,而且经过12月的随访,患者 基本情况良好,无复发或转移的症状与体征。
| [1] | Argani P, Ladanyi M. Renal carcinomas associated with Xp11.2 translocations/TFE3 gene fusions[M]//John N Eble.Pathology and Genetics of Tumors of the Urinary System and Male Genital Organs. Lyon: IARC Press,2004: 37-8. |
| [2] | Argani P, Ladanyi M. Translocation carcinomas of the kidney[J]. Clin Lab Med, 2005, 25(2): 363-78. |
| [3] | Komai Y, Fujiwara M, Fujii Y, et al. Adult Xp11 translocation r ena l c e l l c a r c inoma di agnos ed by cytogene t i c s and immunohistochemistry [J]. Clin Cancer Res, 2009, 15(4): 1170-6. |
| [4] | Argani P, Olgac S, Tickoo SK, et al. Xp11 translocation renal cell carcinoma in adults:expanded clinical, pathologic, and genetic spectrum[J]. Am J Surg Pathol, 2007, 31(8): 1149-60. |
| [5] | Meyer PN, Clark JI, Flanigan RC, et al. Xp11.2 translocation renal cell carcinoma with very aggressive course in five adults[J]. Am J Clin Pathol, 2007, 128(1): 70-9. |
| [6] | Rao Q, Williamson SR, Zhang S, et al. TFE3 breakapart FISH has a higher sensitivity for Xp11.2 translocationociated renal cell carcinoma compared with TFE3 or cathepsin K immunohistochemical staining alone: expanding the morphologic spectrum[J]. Am J Surg Pathol, 2013, 37(6): 804-15. |
| [7] | Srigley JR, Delahunt B, Eble JN, et al. The International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia[J]. Am J Surg Pathol, 2013, 37(10): 1469-89. |
| [8] | Camparo P, Vasiliu V, Molinie V, et al. Renal translocation carcinomas: clinicopathologic, immunohistochemical,and gene expression profiling analysis of 31 cases with a review of the literature[J]. Am J Surg Pathol, 2008, 32(5): 656-70. |
| [9] | Hung CC, Pan CC, Lin CC, et al. XP11.2 translocation renal cell carcinoma:clinical experience of Taipei Veterans General Hospital[J]. J Chin Med Assoc, 2011, 74(11): 500-4. |
| [10] | Wu A, Kunju LP, Cheng L, et al. Renal cell carcinoma in children and young adults:analysis of clinicopathological, immunohistochemical and molecular characteristics with an emphasis on the spectrum of Xp11.2 translocation–associated and unusual clear cell subtypes[J]. Histopathology, 2008, 53(5): 533-44. |
| [11] | Armah HB, Parwani AV. Renal cell carcinoma in a 33-year-old male with an unusual morphology and an aggressive clinical course: possible Xp11.2 translocation[J]. Pathology, 2008, 40(3): 306-8. |
| [12] | Argani P, Antonescu CR, Illei PB, et al. Primary renal neoplasms with the ASPL-TFE3 gene fusion of alveolar soft part sarcoma: a distinctive tumor entity previously included among renal cell carcinomas of children and adolescents[J]. Am J Pathol, 2001, 159(1): 179-92. |
| [13] | Argani P, Ladanyi M. Distinctive neoplasms characterised by specific chromosomal translocations comprise a significant proportion of paediatric renal cell carcinomas[J]. Pathology, 2003, 35(6): 492-8. |
| [14] | Argani P, Ladanyi M. The evolving story of renal translocation carcinomas[J]. Am J Clin Pathol, 2006, 126(3): 332-4. |
| [15] | Argani P, Ladanyi M. Recent advances in pediatric renal neoplasia[J]. Adv Anat Pathol, 2003, 10(5): 243-60. |
 2015, Vol. 42
2015, Vol. 42


