文章信息
- 韩璐,赵真真,王笑峰,李慧,罗兵. 2015.
- HAN Lu, ZHAO Zhenzhen, WANG Xiaofeng, LI Hui, LUO Bing. 2015.
- TLR3和TLR4基因多态性与EBV相关胃癌易感性的关系
- Relationship of TLR3 and TLR4 Gene Polymorphism with Susceptibility of EBVassociated Gastric Carcinoma
- 肿瘤防治研究, 2015, 42(01): 14-18
- Cancer Research on Prevention and Treatment, 2015, 42(02): 14-18
- http://www.zlfzyj.com/CN/10.3971/j.issn.1000-8578.2015.01.004
-
文章历史
- 收稿日期:2014-03-13;
- 修回日期:2014-07-29
2. 266003青岛,青岛大学医学院附属医院检验科
2. Department of Clinical Laboratory, The Affiliated Hospital of Qingdao University Medical College, Qingdao 266003, China
EB病毒(Epstein-Barr virus,EBV)为人类疱疹病毒4型,EBV感染在世界范围内非常普遍,全球98%的人口携带有该病毒,但通常无症状或不发病,是传染性单核细胞增多症的病原体,且与多种肿瘤的发生相关[1]。EBV相关胃癌(EBV-associated gastric carcinoma,EBVaGC)约占胃癌总数的10%左右[2],在胃癌中的比率并不是很高,在不同国家的比例占1.3%至20.1%不等 [3,4],但由于胃癌在世界范围内的高发病率和死亡率,EBVaGC的发病绝对数很高。
Toll样受体家族(TLRs)是一类专门识别各种感染的模式识别受体(pattern recognition receptor,PRR),与感染及恶性肿瘤关系密切。迄今已发现TLRs家族中有11种TLR,Ueta等[5]研究发现TLR3基因有7个单核苷酸多态性位点(single nucleotide polymorphisms,SNPs),该基因多态性对肿瘤的发生有重要影响。TLR4基因存在多个SNPs,其Asp299Gly是错义突变位点,位于第4外显子,可启动强烈的抗肿瘤细胞免疫,因此其突变与肿瘤的关系亟待验证。
TLRs的遗传差异对疾病有重要影响,而EBV感染在EBVaGC发生中的作用一直是人们关注的热点,因此,探讨TLRs在EBVaGC发生机制中的作用具有实际意义。本研究首次对EBVaGC组织中TLRs的TLR3及TLR4 基因多态性进行检测,以期明确其多态性与EBVaGC发生是否存在相关性。 1 资料与方法1.1 研究对象
胃癌组:采用原位杂交检测胃癌石蜡组织切片中EBV编码的小分子非多聚腺苷酸EBER1的转录,筛选出41例EBV阳性胃癌,同时选取临床病理特征与之匹配的62例EBV阴性胃癌(EBVnegative gastric carcinoma,EBVnGC)作为研究对象,EBV阳性与EBV阴性胃癌组间种族、年龄、性别、病理分期及浸润程度差异无统计学意义 (P>0.05)。对照组为青岛大学医学院附属医院健康体检者64例,采集空腹EDTA抗凝静脉血5 ml分离外周血单个核细胞。种族、年龄和性别在胃癌组与对照组间比较差异无统计学意义。1.2 组织细胞DNA提取
采用QIAamp DNA FFPE试剂盒(德国Qiagen公司)提取石蜡包埋胃癌组织DNA,具体操作步骤按试剂盒说明书进行;采用酚-氯仿-异戊醇法常规提取新鲜胃癌组织和外周血单个核细胞DNA,干燥后以TE缓冲液(pH 8.0)溶解DNA,4℃保存备用。1.3 TLR3c.1377和TLR4基因Asp299Gly多态性的检测
采用PCR-RFLP技术检测胃癌组织和健康对照外周血细胞DNA TLR3c.1377以及TLR4Asp299Gly多态性,所用引物及限制性内切酶见表 1。 族成员的多个SNPs、独立因素的进一步分析加以证实。
 |
TLR3 c.1377基因SNP为TLR3受体羧基端1377位碱基C突变为T;TLR4 Asp299Gly SNP是编码TLR4受体第299个氨基酸的密码子由GAT→GGT,天冬氨酸(Asp)代替甘氨酸(Gly)。 1.3.1 TLR3基因TLR3c.1377多态性检测
PCR反应体系为30 μl,包括10 ×Buffer 3 μl,dNTPs 2.4 μl(终浓度0.2 mM),上下游引物各0.9 μl(终浓度0.3 μM),Taq DNA聚合酶0.24 μl(终浓度0.04 mM),DNA 模板2 μl,无酶双蒸水20.56 μl。循环条件为94℃预变性5 min;然后94℃ 30 s,55℃ 30 s,72℃ 30 s,共35个循环;最后72℃延伸10 min。取3 μl PCR扩增产物于含溴化乙锭(0.5 µg/ml)的2%琼脂糖凝胶中电泳,凝胶成像系统记录电泳结果。每次PCR反应均用无菌双蒸水作阴性对照。
采用限制性内切酶TaqⅠ(TthHB8 Ⅰ)酶切上述TLR3(c.1377C/T)基因多态性PCR产物,酶切体系20 μl,包括10×Taq ⅠBasal Buffer 2 μl,10 u/μl TaqⅠ1 μl,0.1% BSA 2 μl,PCR产物10 μl,无酶双蒸水5 μl。混匀后瞬时离心,65℃水浴30 min。酶切产物于含溴化乙锭(0.5 µg/ml)的2%琼脂糖凝胶中电泳,凝胶成像系统记录实验结果。限制性内切酶 TaqⅠ识别序列为T↓CGA。
PCR产物酶切后电泳条带仍为337 bp者为纯合突变型TT基因型;酶切产物电泳观察到274 bp和63 bp两条带者为纯合野生型CC基因型;PCR产物酶切后既出现274 bp和63 bp两条带,同时保留有337 bp条带者为杂合CT基因型。1.3.2 TLR4基因Asp299Gly多态性检测
TLR4基因Asp299Gly多态性PCR反应体系与TLR3c.1377多态性检测相同。采用限制性内切酶NcoⅠ酶切上述PCR产物,酶切体系20 μl,包括10×K Buffer 2 μl,10 u/μl NcoⅠ1 μl,PCR产物10 μl,无酶双蒸水7 μl。混匀后瞬时离心,37℃水浴30 min。酶切产物于含溴化乙锭(0.5 µg/ml)的2%琼脂糖凝胶中电泳,凝胶成像系统记录实验结果。
PCR产物酶切后电泳条带仍为140 bp者系野生型Asp/Asp等位基因型;PCR产物酶切后出现77 bp和63 bp两条带者为突变型Gly/Gly基因型;PCR产物酶切后出现77 bp、63 bp和140 bp三条带者为Asp/Gly基因型。1.4 PCR产物测序
将上述部分标本PCR产物30 μl送北京华大基因有限公司采用末端终止法进行双向测序。完成后,用Chromas软件查看峰图文件,DNAStar软件(Larsergene,version 7.0)进行序列剪接和对比分析,并与基因库中相应的TLRs野生型DNA序列进行对比分析,以验证PCR-RFLP鉴定结果。1.5 统计学方法
采用SPSS 17.0 统计软件分析。基因型和等位基因频率在各组中分布差异用χ2检验;采用非条件Logistic 回归计算比值比(odds ratio,OR)及P值表示各基因型胃癌风险的关联性。P≤0.05为差异有统计学意义。2 结果2.1 TLR3c.1377基因多态性检测分析结果
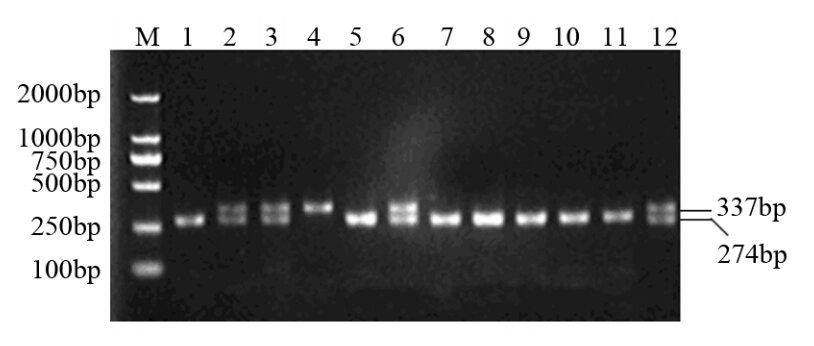
103例胃癌组织DNA和64例健康对照外周血DNA均扩增出TLR3c.1377基因337 bp特异性条带,部分标本PCR产物TaqⅠ酶切后电泳结果见图 1。
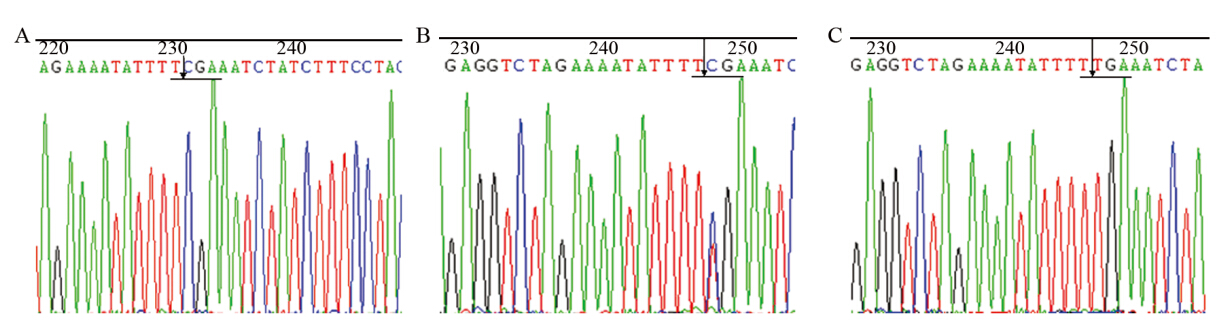
选取上述部分CC、TT和CT基因型标本PCR产物进行测序分析,测序结果与GenBank TLR3标准型(野生型)DNA序列比较,显示与PCR-RFLP鉴定结果一致。3种基因型各1例标本测序结果见图 2。
|
| M: PCR marker DL2000; 1,5,7-11: CC genotype; 2,3,6,12: CT genotype; 4: TT genotype图 1 部分标本TLR3c.1377基因分型PCR产物TaqⅠ酶切电泳结果 Figure 1 The electrophoresis results of TaqⅠrestriction endonuclease digestion after PCR for TLR3c.1377 genotyping in part of specimens |
|
| A: representative samples (CC genotype) which possess TaqI site T↓CGA and the 1377bp is still C; B: representative samples (CT genotype) which possess TaqI site T↓CGA and overlapped peak; C: representative samples (TT genotype) which lack of Taq Ⅰ restriction site and the 1377bp changes into T. Arrow indicates the Taq Ⅰ restriction site图 2 TLR3 ( c.1377C/T ) PCR产物测序结果 Figure 2 PCR products sequence analysis of TLR3 (c.1377C/T) |
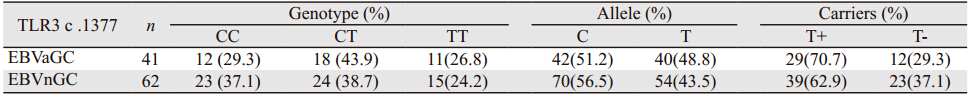
对上述TLR3c.1377基因多态性检测结果进行分析显示,胃癌组与对照组之间TLR3c.1377基因型频率差异有统计学意义(χ2=7.36,P=0.025)。胃癌组T等位基因频率高于对照组(46.5% vs.29.7%,χ2=8.40,P=0.004)。胃癌组T等位基因携带者的频率高于对照组(66.0% vs.45.3%,χ2=6.96,P=0.008,OR=2.435,95% CI: 1.238~4.443),见表 2。
 |
进一步比较EBVaGC和EBVnGC组织中TLR3 c .1377基因多态性差异。统计分析显示两组间TLR3 c .1377基因型频率(χ2= 0 .6 7 6,P=0.713)、T等位基因频率(48.8% vs. 43.5%,χ2=0.545,P=0.461)及T等位基因携带者的频率(70.7% vs. 62.9%,χ2=0.674,P=0.412)差异无统计学意义,见表 3。
 |
103例胃癌组织DNA和64例健康对照外周血DNA均扩增出TLR4Asp299Gly 140 bp特异性条带,采用限制性内切酶NcoⅠ酶切PCR产物。
本研究中所有胃癌患者和健康对照组PCR产物酶切后,其电泳条带仍为140 bp,即均为野生型Asp/Asp基因型(χ2=0,P>0.05),表明胃癌患者和健康对照组TLR4 Asp299Gly位点均未出现A→G突变。部分标本PCR产物的酶切电泳结果见图 3。
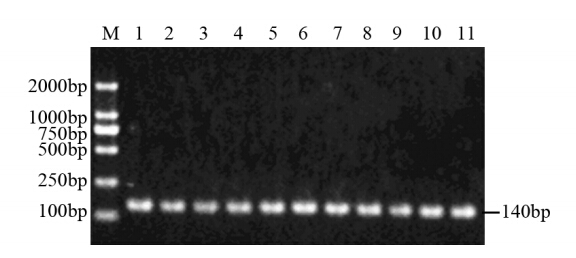
|
| M: PCR marker DL2000; 1-11: wild type Asp/Asp genotype图 3 部分标本TLR4 Asp299Gly基因分型PCR产物NcoⅠ酶切电泳结果 Figure 3 The electrophoresis results of NcoⅠrestriction endonuclease digestion after PCR for TLR4 genotyping in part of specimens |
TLR家族成员广泛分布在免疫细胞、正常细胞及恶性上皮细胞中,某些成员与宿主感染应答及肿瘤的发生关系密切,人类约20%的恶性肿瘤与病毒感染相关,TLRs编码基因多态性可能参与多种病毒相关肿瘤的发生。研究表明,TLR3编码基因多态性与多种肿瘤如乳腺癌、口腔鳞状细胞癌、HBV相关肝细胞癌等的发病和预后相关[6,7,8]。本研究TLR3c.1377基因多态性检测结果表明,胃癌患者中,TT基因型频率和T等位基因频率均明显高于对照组,提示T等位基因为胃癌发病的危险因子,携带T等位基因会增加胃癌的易感性,而C等位基因是一个保护基因。
Omrane等[9]报道,在北非突尼斯人群中TLR4基因Asp299Gly与大肠癌晚期淋巴结转移有关,并且能促进疾病的进展。一项克什米尔种族研究表明,TLR4Asp299Gly基因多态性与胃癌发病相关,并且对于胃远侧部癌变更有意义[10]。而亚洲多个地区的有关研究表明,TLR4 Asp299Gly基因多态性与膀胱癌、肺癌、结肠癌的发生发展无明显相关性[11,12,13]。但Lorenz等研究证实芬兰人TLR4基因Asp299Gly突变型高达9.5%[14]。以上研究结论提示此基因多态性存在种族差异。本研究结果显示,TLR4Asp299Gly在EBVaGC组、EBVnGC组及对照组均未检测到该位点多态性。
EBV既是感染因子,又是重要的DNA肿瘤病毒,非特异性免疫如干扰素能够在特异性免疫系统发挥作用前抑制病毒复制,而TLRs能够参与由干扰素所激活的抗病毒信号通路,因此推测TLRs基因多态性可能与EBVaGC易感性具有一定关联。
TLRs家族与EBV相关鼻咽癌(nasopharyngeal carcinoma,NPC)的研究多见,Moumad等[15]检测了北非492例NPC患者及373例对照人群5种PRR的26个SNPs,分析发现其基因变异与NPC易感性相关,其中TLR3 rs3775291的SNP与EBV相关NPC关系最大,每个风险等位基因会增加18%发病风险。另外一项研究证实,TLR3基因多态性与中国广东地区EBV相关NPC发病易感性相关,该研究选取434例NPC患者及512例健康对照者,利用直接测序法检测TLR3的4个SNPs,结果显示TLR3基因829A/C与NPC易感性相关,C等位基因为NPC的危险因子[16]。
本研究显示TLR3c.1377多态性与EBVaGC易感性无明显相关性,EBVaGC有其独特性,与其他EBV相关肿瘤如NPC、Burkitt's淋巴瘤等组织中病毒编码基因的表达不完全相同,如公认的EBV细胞转化基因即潜伏膜蛋白(latent membrane protein,LMP1)编码基因在EBVaGC中不表达。且Valente等[17]研究证实TLR7能够诱发EBV LMP1的表达,并在TLR3和TLR9的协同作用下刺激表达LMP1的细胞产生IFNs,成为红斑狼疮的诱发因素。表明部分TLR 家族成员可通过激活LMP1,进而产生一系列相关细胞因子对疾病产生影响。而EBVaGC中EBV属于Ⅰ型潜伏,不表达LMP1,因此TLR3不能通过LMP1相关信号通路在EBVaGC发生发展中发挥作用,进而无法对EBVaGC易感性产生影响。
总之,本研究证实TLR3c.1377基因型频率、等位基因频率与胃癌发生有关,携带T等位基因会增加患胃癌的风险,TLR3c.1377基因多态性与胃癌组织中EBV感染无相关性。EBV感染与TLRs家族其他成员多态性在EBVaGC中的相互关系有待更大样本的研究,包括多个TLRs家
| [1] | Münz C, Moormann A. Immune escape by Epstein-Barr virus associated malignancies[J]. Semin Cancer Biol, 2008, 18(6): 381-7. |
| [2] | Fukayama M, Chong JM, Kaizaki Y. Epstein-Barr virus and gastric carcinoma[J]. Gastric Cancer, 1998, 1(2):104-14. |
| [3] | Uozaki H, Fukayama M. Epstein-Barr virus and gastric carcinomaviral carcinogenesis through epigenetic mechanisms[J]. Int J Clin Exp Pathol, 2008, 1(3): 198-216. |
| [4] | Lee JH, Kim SH, Han SH, et al. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: a meta-analysis[J]. J Gastroenterol Hepatol, 2009, 24(3): 354-65. |
| [5] | Ueta M, Sotozono C, Inatomi T, et al. Toll- like receptor 3 gene polymorphisms in Japanese patients with Stevens-Johnson syndrome[J]. Br J Ophthalmol, 2007, 91(7): 962-5. |
| [6] | Yeyeodu ST, Kidd LR, Oprea-Ilies GM, et al. IRA-K4 and TLR3 Sequence Variants may alter breast cancer risk among AfricanAmerican women[J]. Front Immunol, 2013, 4: 338. |
| [7] | Li G, Zheng Z. Toll-like receptor 3 genetic variants and susceptibility to hepatocellular carcinoma and HBV-related hepatocellular carcinoma[J].Tumour Biol, 2013, 34(3): 1589-94. |
| [8] | Zeljic K, Supic G, Jovic N, et al. Association of TLR2,TLR3,TLR4 and CD14 genes polymorphisms with oral cancer risk and survival[J].Oral Dis, 2014, 20(4): 416-24. |
| [9] | Omrane I, Baroudi O, Kourda N, et al. Positive link between variant Toll-like receptor 4 (Asp299Gly and Thr399Ile) and colorectal cancer patients with advanced stage and lymph node metastasis[J]. Tumour Biol, 2014, 35(1): 545-51. |
| [10] | Qadri Q, Rasool R, Afroze D, et al. Study of TLR4 and IL-8 gene polymorphisms in H.pylori-induced inflammation in gastric cancer in an ethnic Kashmiri population[J]. Immunol Invest, 2014, 43(4): 324-36. |
| [11] | Singh V, Srivastava N, Kapoor R,et al. Single-nucleotide polymorphisms in genes encoding toll-like receptor -2, -3, -4, and -9 in a case-control study with bladder cancer susceptibility in a North Indian population[J]. Arch Med Res, 2013, 44(1): 54-61. |
| [12] | Xie ZQ, Lv B, Zou LJ, et al. Genotyping of toll-like receptor 4 in Hubei population of Chinese[J]. Huazhong Ke Ji Da Xue Xue Bao(Yi Xue Ban), 2004, 33(5): 630-2. [谢志强, 吕斌, 邹立君, 等. 湖北人群Toll样受体4基因多态性研究[J].华中科技大学学报(医学版), 2004, 33(5): 630-2.] |
| [13] | Okayama N, Fujimura K, Suehiro Y, et al. Simple genotype analysis of the Asp299Gly polymorphism of the Toll-like receptor-4 gene that is associated with lipopolysaccharide hyporesponsiveness[J]. J Clin Lab Anal, 2002, 16(1): 56-8. |
| [14] | Lorenz E, Hallman M, Marttila R, et al. Association between the Asp299Gly polymorphisms in the Toll-like receptor 4 and premature births in the Finnish population[J]. Pediatr Res, 2002, 52(3): 373-6. |
| [15] | Moumad K, Lascorz J, Bevier M, et al. Genetic polymorphisms in host innate immune sensor genes and the risk of nasopharyngeal carcinoma in North Africa[J]. G3 (Bethesda), 2013, 3(6): 971-7. |
| [16] | He JF, Jia WH, Fan Q, et al. Genetic polymorphisms of TLR3 are associated with Nasopharyngeal carcinoma risk in Cantonese population[J]. BMC Cancer, 2007, 7: 194. |
| [17] | Valente RM, Ehlers E, Xu D, et al. Toll-like receptor 7 stimulates the expression of Epstein-Barr virus latent membrane protein 1[J]. PLoS One, 2012, 7(8): e43317. |
 2014, Vol. 42
2014, Vol. 42