Visualization Analysis of Research Hotspots and Trends in Treatment of Radioactive Iodine Refractory Differentiated Thyroid Carcinoma
-
摘要:目的
探讨2004—2024年放射性碘难治性甲状腺癌(RAIR-DTC)治疗领域的相关研究热点及未来发展趋势。
方法从Web of Science中检索2004年1月—2024年5月关于RAIR-DTC治疗领域的文献。采用CiteSpace、VOSviewer、Microsoft Office Excel软件对发文量、国家、机构、作者、关键词和共引网络进行可视化分析及总结。
结果最终纳入了677篇文献。国家和机构共现分析显示美国及美国德克萨斯大学安德森癌症中心在该领域最具生产力和影响力。作者和引文共现分析显示Schlumberger M和Brose MS对相关领域贡献突出。高频关键词和关键词聚类显示酪氨酸激酶抑制剂和疾病预后因素的探究是当前的研究热点。结合关键词突现分析显示通过高质量临床试验提供的可靠数据优化临床获益,实现个体化、精准化治疗管理将成为未来的研究趋势。
结论靶向药物对于RAIR-DTC治疗拥有广阔的应用前景,关注疾病预后的预测因素为医学实践提供了重要指导。
-
关键词:
- 放射性碘难治性甲状腺癌 /
- 治疗 /
- CiteSpace /
- VOSviewer /
- 文献计量学
Abstract:ObjectiveTo explore research hotspots and future development trends in radioactive iodine refractory differentiated thyroid carcinoma (RAIR-DTC) treatment from 2004 to 2024.
MethodsLiterature on RAIR-DTC treatment published from January 2004 to May 2024 was retrieved from the Web of Science (WOS) database. CiteSpace, VOSviewer, and Microsoft Office Excel were used for visual analysis of publication volume, countries, institutions, authors, keywords, and co-citation networks.
ResultsA total of 677 articles were included in the analysis. National and institutional co-occurrence analysis revealed that the United States, along with the MD Anderson Cancer Center at the University of Texas, was the most productive and influential in this field. Author and citation co-occurrence analysis highlighted the substantial contributions of Schlumberger M and Brose MS to the field. The exploration of high-frequency keywords and keyword clustering indicated tyrosine kinase inhibitors and disease prognostic factors were current research hotspots. Keyword burst analysis suggested that future research trends would focus on optimizing clinical benefits through reliable data provided from high-quality clinical trials and achieving personalized, precise treatment management.
ConclusionTargeted drugs hold remarkable potential for RAIR-DTC treatment, and emphasizing predictive factors for disease prognosis offers valuable guidance for medical practice.
-
0 引言
分化型甲状腺癌(Differentiated thyroid carcinoma, DTC)占甲状腺癌的90%以上,其中部分肿瘤细胞失分化后其钠碘同向转运体(Sodium iodine isotropic transporter, NIS)的表达及功能出现异常,最终发展为放射性碘难治性分化型甲状腺癌(Radioactive iodine refractory differentiated thyroid carcinoma, RAIR-DTC)[1],RAIR-DTC进展迅速、病死率高[2]。近年来相关研究增多,积累了大量文献,但这些研究成果尚未得到系统分析。文献计量学分析使用数学和统计方法对特定研究领域进行定性和定量分析,从客观视角为读者提供该领域发展趋势和前沿热点[3-4]。本研究采用 VOSviewer、CiteSpace 软件对近20年来RAIR-DTC治疗相关研究进行分析,旨在阐明该领域的研究概况和发展趋势,为今后的研究工作提供新思路。
1 资料与方法
1.1 数据来源及检索策略
计算机系统检索Web of Science Core Collection(WOSCC)核心合集数据库。检索时限为 2004年1月—2024年5月。本研究使用的检索策略为: TS=(Radioactive iodine-refractory differentiated thyroid cancer OR Radioactive iodine refractory thyroid cancer OR radioiodine-refractory differentiated thyroid cancer OR Radioactive iodine refractory thyroid cancer OR Radioiodine refractory differentiated thyroid carcinoma OR Radioiodine refractory advanced DTC OR Radioiodine-Refractory Thyroid Cancer OR RRDTC OR RAIR-DTC)AND TS=(Therapeutics OR Therapeutic OR Therapy OR Therapies OR Treatment OR Treatments)。文章类型只选择了“article”和“review”,语言仅限于“English”。经筛选及去重,最终纳入文献677篇,导出完整记录和参考文献,将其以“download_txt”格式保存。
1.2 统计学方法
采用CiteSpace 6.3.1 Advanced通过对数似然率算法行关键词聚类分析,并通过Kleinberg突发检测算法行关键词突现分析。使用VOSviewer1.6.20绘制机构合作网络图谱,对国家、机构、共被引分析(作者和参考文献)进行共现分析后汇总统计结果。使用Microsoft Office Excel 2019显示历年的发文量。
2 结果
2.1 年发文量分析
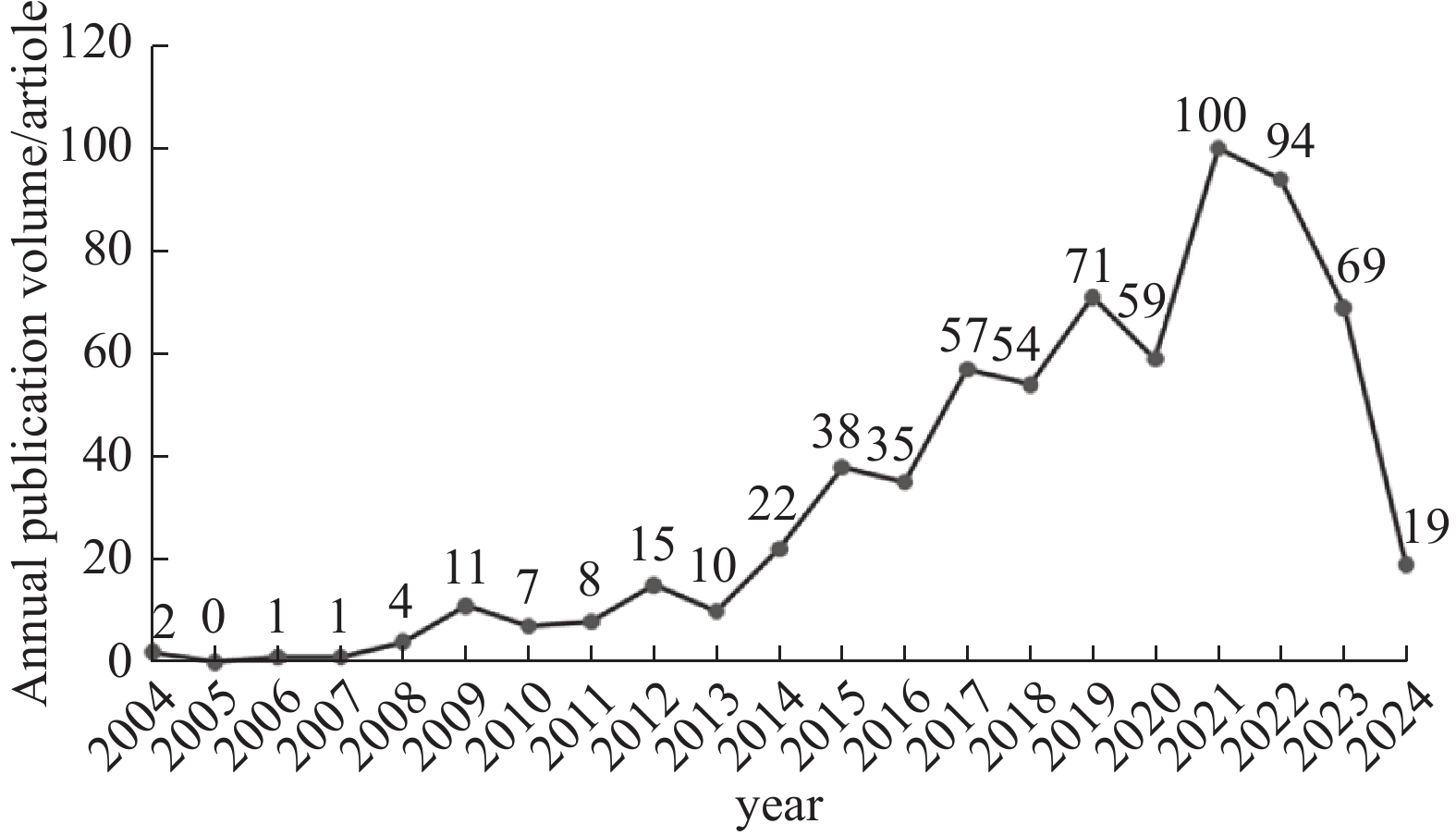
2004—2014年属于缓慢增长阶段,表明该领域尚处于起步阶段;2014年后论文数量增速加快,2021年发文量达到最高(100篇,占总发文量的14.77%)。RAIR-DTC治疗领域知识量总体呈增长态势,反映出相关研究热度正逐渐升高,见图1。
2.2 国家/地区和机构分析
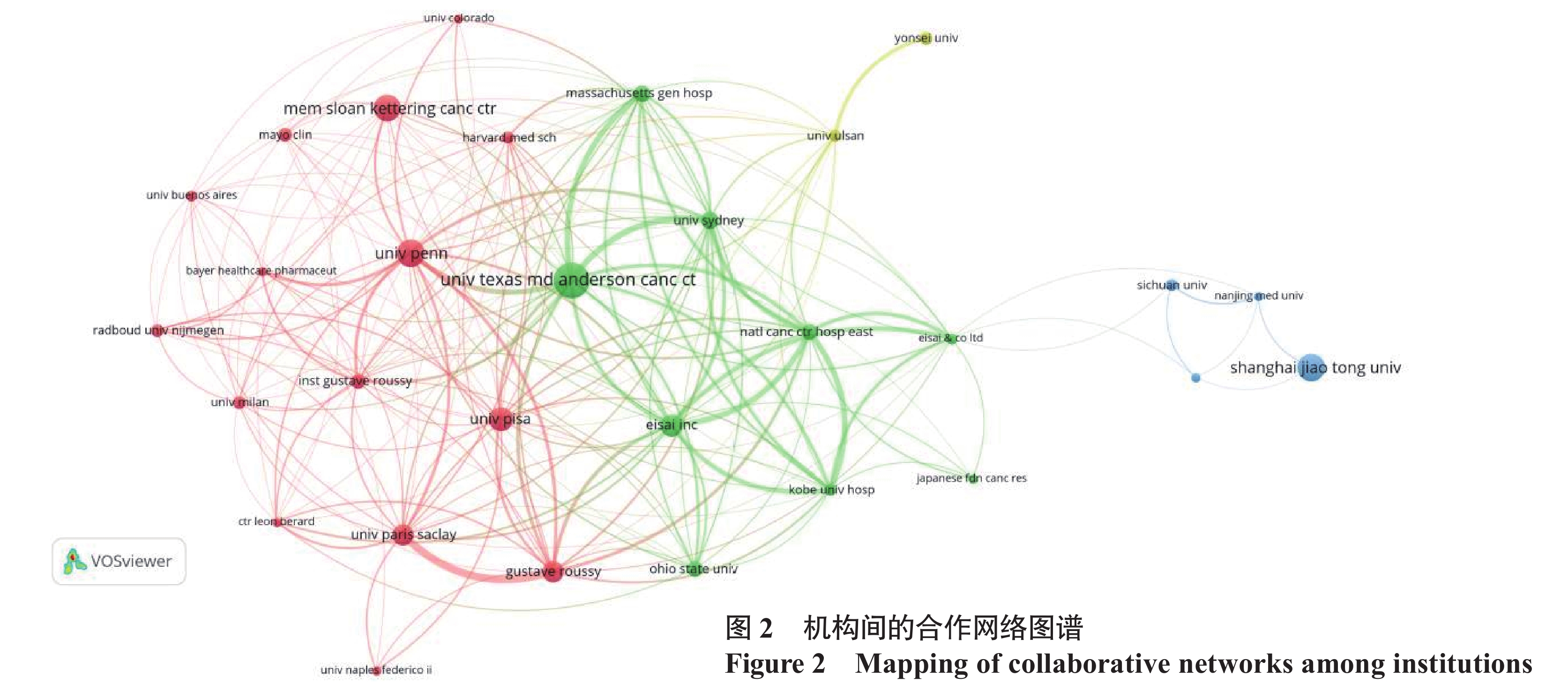
图2中节点大小表示机构的发文量,节点越大表示发文量越多;节点的颜色表示机构所属的聚类;节点之间的线条表示存在合作关系,线条越粗表示关系越紧密。目前有56个国家/地区、1 092个机构参与677篇研究的发表,美国发文量全球第一(203篇,占总发文量的29.99%),美国发文的总被引频次也远超其他国家(11 416次)。美国德克萨斯大学安德森癌症中心发文量(36篇)和被引频次(4 380次)位居第一。中国发文量位居第二(129篇),其中上海交通大学在该领域研究较多(28篇)。分析发现中国机构与其他国家的机构合作较少。
2.3 作者与共被引作者分析
该领域研究涉及3 710名作者和9 660名共被引作者。Schlumberger M发文量最多(25篇),其次为Brose MS(22篇)和Leboulleux S(22篇);共被引次数最多的作者为Brose MS(681次)和Schlumberger M(681次),其次为Durante C(359次),见表1。Schlumberger等[5-6]的研究主要涉及仑伐替尼(Lenvatinib)治疗RAIR-DTC的疗效评估及药物作用分子机制。此外,该作者还关注了RAIR-DTC患者伴骨转移行Radium-223的疗效评估,但因治疗反应不佳已被终止[7]。Brose等[8-11]的研究主要涉及多种靶向药物的疗效和相关药代动力学。
表 1 发文量与被引频次排名前10的作者Table 1 Top 10 authors in terms of publication volume and citation frequencyRanking Authors Number of publications (articles) Authors Cited frequency (times) 1 Schlumberger M 25 Brose MS 681 2 Brose MS 22 Schlumberger M 681 3 Leboulleux S 22 Dutcus C 359 4 Elisei R 20 Cabanillas ME 340 5 Wirth LJ 16 Haugen BR 328 6 Tahara M 15 Xing M 324 7 Capdevila J 15 Sherman SI 232 8 Chen LB 15 Bieber KC 175 9 Lin Y 15 Ho AL 158 10 Cabanillas ME 14 Leboulleux S 150 2.4 引用参考文献分析
高被引文献代表了该领域的知识基础。被引次数排在前10的参考文献见表2。被引频次最多的文献是Lenvatinib versus placebo in radioiodine-refractory thyroid cancer(Schlumberger等,2015),被引340次,该文是一项针对RAIR-DTC进行的多中心临床Ⅲ期试验,结果示仑伐替尼组的中位无进展生存期(Progression-free survival, PFS)、客观缓解率(Overall response rate, ORR)均显著高于安慰剂组(18.3个月vs. 3.6个月,64.8% vs. 1.5%)[12]。其次是Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial(Brose等,2014),被引326次,该文示索拉非尼组的RAIR-DTC患者中位PFS、ORR均显著高于安慰剂组(10.8个月vs.5.8个月,12.2% vs. 0.5%),且甲状球蛋白(Thyroglobulin, Tg)水平显著降低[13]。被引频次排名第3的是Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limitations of radioiodine therapy(Durante等,2006),被引288次,该研究指出131I治疗应持续到任何异常摄取消失或累积活度达到22 GBq(600 mci)[2]。
表 2 被引次数排在前10的参考文献Table 2 Top 10 references in terms of citationsRanking Title of literature Journal First Author Publication Number of
citations
(times)1 Lenvatinib versus placebo in radioiodine-refractory thyroid cancer New England Journal of Medicine Schlumberger M 2015 340 2 Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomized, double-blind, phase 3 trial. Lancet Brose MS 2014 326 3 Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limitations of radioiodine therapy. Clinical Endocrinology Durante Dutcus C 2006 288 4 2015 American Thyroid Association ManagementGuidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and DifferentiatedThyroid Cancer. Thyroid Haugen BR 2016 274 5 Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. New England Journal of Medicine Ho AL 2013 149 6 Phase Ⅱ trial of sorafenib in advanced thyroid cancer. Clinical Gupt-Abramson V 2008 105 7 Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic-differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Bieber KC 2010 104 8 Definition and management of radioactive iodine-refractory differentiated thyroid cancer Lancet Diabetes Endo Schlumberger M 2014 101 9 Integrated genomic characterization of papillarythyroid carcinoma Cell Agrawal N 2014 99 10 New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1) European Journal of Cancer Eisenhauer E 2009 99 2.5 高频关键词分析
文献关键词是对文献内容的高度凝练,反映了文献的研究主题及中心思想,中心度可以反映节点在整个网络中的媒介能力和重要程度,对高频次、高中心度的关键词分析有助于挖掘该领域的研究热点[14],在排除研究对象类高频关键词后,频次较高的关键词为“tyrosine kinase inhibitor” “double blind” “sorafenib”,中心度较高的关键词为“kinase inhibitors” “angiogenesis” “survival”。可见该领域研究主要集中在酪氨酸激酶抑制剂和疾病预后生存。
2.6 关键词聚类分析
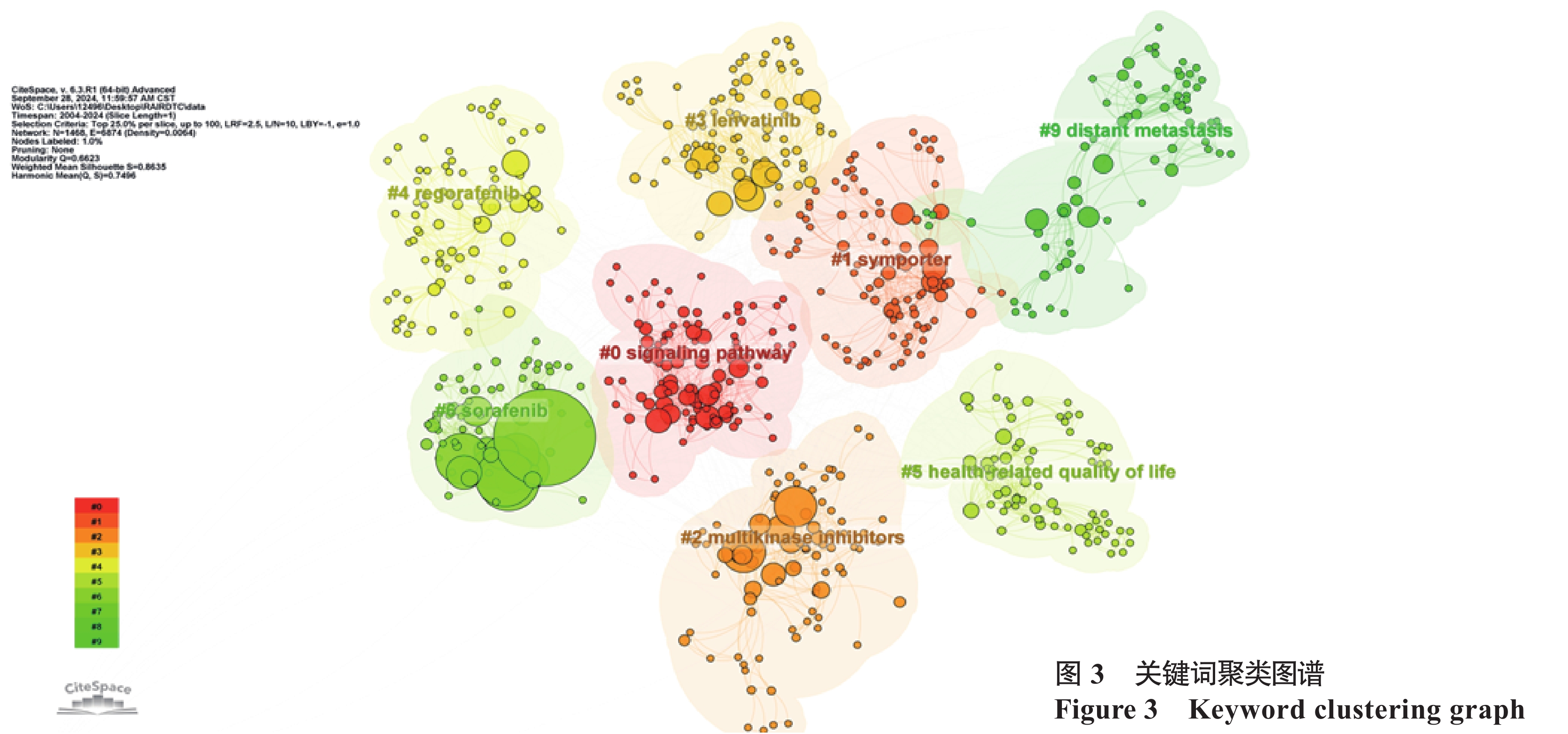
关键词聚类分析是通过把共现关系明显的关键词,利用聚类统计方法进行简化分类,展示研究的重点[14]。采用对数似然率算法进行聚类分析后得出图3,图中相近的节点通常代表主题上或概念上相近的关键词,不同的颜色编码代表不同的关键词集群,去除与文章研究内容无关和重复的聚类后,共得到8个主要聚类,聚类模块值Q为0.66(>0.30),表明聚类结构显著;平均轮廓值S为0.86(>0.70),表明聚类结果可信度高[14]。进一步对以上几个主要聚类进行详细分析:(1)“#0 signaling pathway” “#1 symporter” “#2 multikinase inhibitors”“#3 levatinib” “#4 regorafenib” “#5 sorafenib”包含signaling pathway、symporter、multikinase inhibitors、levatinib、 regorafenib、braf v600e mutation、sorafenib等关键词,可归纳为RAIR-DTC致病机制及与机制对应的治疗,治疗方式中酪氨酸激酶抑制剂占比较大。(2)“#6”、“#9”包含health-related quality of life、adverse effects、distant metastasis、prognostic factor等关键词,可归纳为RAIR-DTC的疾病预后及影响因素。
2.7 关键词突现分析
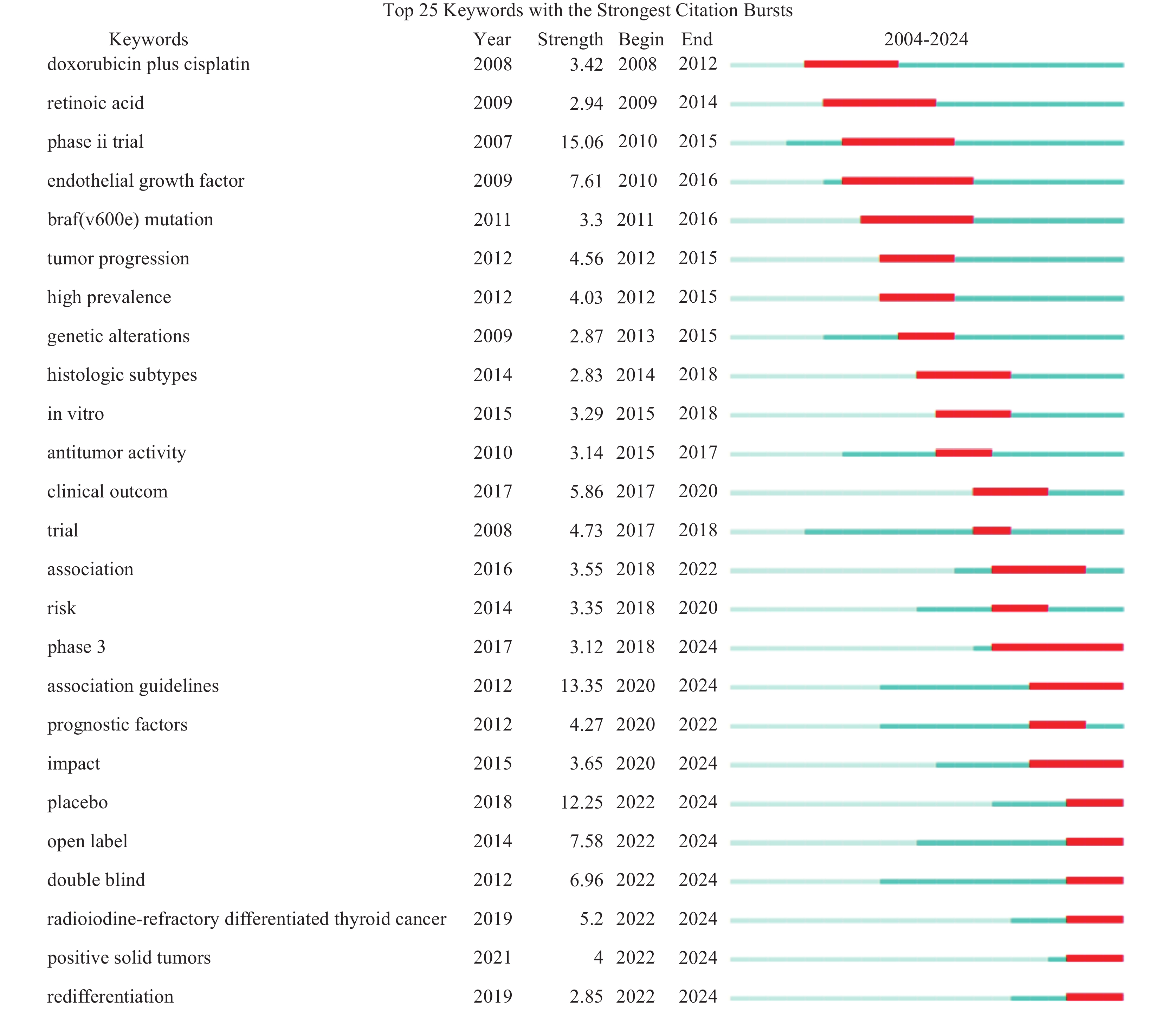
关键词突现分析某个时期内频次变化率高的词,尤其延续至今的关键词,反映了新兴趋势和热点问题[15]。图4中Year表示关键词出现时间,Strength表示突现强度,Begin表示突现开始的年份,End表示突现结束的年份,深蓝色条表示关键词开始出现,红色条表示突现时间段。依照突现性较高的关键词分析研究热点的演变,可见RAIR-DTC治疗早期采用细胞毒性化学疗法(doxorubicin plus cisplatin),但其不良反应大、反应率低。维甲酸类药物(retinoic acid)是甲状腺癌再分化研究最早用到的药物之一,通过作用于维甲酸、视黄酸受体等核受体提高甲状腺癌细胞的摄碘率,但较少能实现有意义的临床获益[16]。随着时间推移,针对RAIR-DTC发生发展相关分子机制的研究不断深入(braf(v600e) mutation、endothelial growth factor,in vitro等),各种分子靶向药物层出不穷,因此伴随着大量的多期临床试验验证其有效性和安全性,图4中phase3、impact、placebo、double blind、redifferentiation等关键词是近年出现并突现至今,针对这些关键词的相关研究可能是RAIR-DTC治疗领域未来研究的重点和方向。
3 讨论
3.1 研究概况
RAIR-DTC治疗领域文献数量逐渐增加,这一趋势得益于治疗手段的飞速发展。美国在此领域处于领先地位,我国具有学术影响力的研究机构如上海交通大学等具有一定发展潜力,但与其他国际机构合作较少。Schlumberger M和Brose MS两位专家在该领域贡献突出,他们在靶向药物的机制研究和临床应用领域作出了较大的贡献。
3.2 研究热点
根据高频词共现分析和聚类分析可观察到RAIR-DTC治疗领域的研究热点主要为酪氨酸激酶抑制剂(Tyrosine kinase inhibitors,TKI)和疾病预后。目前正式发布了较多的临床试验结果的TKI分别是索拉菲尼(Sorafenib)、仑伐替尼(Lenvatinib)、卡博替尼(Cabozantinib)和阿帕替尼(Apatinib)。这四种TKIs均能抑制血管内皮生长因子受体(Vascular endothelial growth factor receptor, VEGFR),从而抑制RET/RAS/RAF/MEK/MAPK和PI3K/AKT/mTOR两个信号通路,限制肿瘤细胞生长[17]。索拉非尼和仑伐替尼是治疗RAIR-DTC的一线用药。索拉非尼可以阻断VEGFR-2和VEGFR-3的活性以抑制肿瘤血管生成,而VEGFR主要表达于心、肺、肾等软组织,骨中表达较少且骨的结构组成较为复杂[13,18-19] ,靶向药物作用困难[20-21],故其对肺转移患者的疗效优于骨转移患者[22]。仑伐替尼是一种多靶点TKI,可抑制VEGFR1-3、成纤维细胞生长因子受体1-4(FGFR1-4)、RET和血小板衍生生长因子受体α(PDGFRα),具有良好的中位PFS、ORR以及可控的不良反应[22–30]。卡博替尼用于治疗12岁及以上患有局部晚期或转移性DTC的成人和儿童患者,也用于一线治疗失败的患者[10,31]。阿帕替尼是高选择性细胞内VEGFR-2的ATP结合位点阻滞剂,可阻断RAF/MEK/ ERK(介导内皮细胞增殖)、p38/MAPK(介导内皮细胞迁移)、PI3K/AKT/mTOR(与内皮细胞存活和血管通透性增加相关)等多种途径,有效抑制肿瘤血管生成[32]。但其导致的3级及以上不良事件的发生率高达73.9%[33],且相关研究多局限于中国,所以需进一步进行不同种族基因组疗效差异、药物不良反应和剂量改良方案的研究。其他有关抑制RAIR-DTC致病基因调控的药物研究也在逐步推进。恩曲替尼(Entrectinib)、拉罗替尼(Larotrectinib)是原肌球蛋白受体激酶(Tropomyosin-related kinase,TRK)的抑制剂,在欧洲已被批准用于治疗NTRK融合基因阳性的DTC患者[33]。赛普替尼(Selpercatinib)、普拉替尼(Pralsetinib)可抑制多种RET变异[34-35]。安罗替尼(Anlotinib)能够有效抑制VEGFR、PDGFR、纤维母细胞生长因子受体(Fibroblast growth factor receptor,FGFR)、c-Kit激酶,从而抗肿瘤血管生成和抑制肿瘤生长[36]。Sun等[37]报道既往MKIs耐药的患者,使用安罗替尼可以在早期控制疾病进展。维罗非尼(Vemurafenib)[38]、舒尼替尼(Sunitinib)、索凡替尼(Surufatinib)也在进行用于治疗RAIR-DTC患者的尝试[39-40]。
疾病预后的研究可以帮助医生对RAIR-DTC患者进行预后评估,选择更有效的治疗策略[41]。Kim等[42]发现高中性粒细胞与淋巴细胞比率、低淋巴细胞与单核细胞比率和高血小板与淋巴细胞比率(Platelet-to-lymphocyte ratio,PLR)与较短的OS显著正相关,这可能与炎性反应因子能抑制免疫、促进肿瘤免疫逃逸有关。高PLR提示凝血系统激活,这为肿瘤提供了额外的营养和保护,从而有利于其生长和转移。Sgrò等发现血清Tg的变化与影像学反应无关,TgAb水平的减少与影像学上对仑伐替尼的治疗反应呈正相关[43]。东部肿瘤协作组(Eastern Cooperative Oncology Group,ECOG)评分(用于评估癌症患者日常活动能力的量表)[44]、靶向治疗后一周的PET/CT最大标准摄取值、VEGF、酪氨酸激酶2(Tyrosine kinase 2,TYK-2)和血管生成素2(Angiopoietin-2,Ang-2)等生物标志物,均在不同研究中体现出对RAIR-DTC患者靶向治疗的预后有一定预测效果[45-47]。探索更简单实用的参数评估预后、确定个体化诊疗的最佳治疗时机和方案,对于提高RAIR-DTC患者的生存率和生活质量至关重要。
3.3 研究趋势
关键词突现分析结果显示了较多有关临床试验研究的词汇,例如该领域国内外研究人员近期发表的重要文献:Leboulleux等[48]一项Ⅱ期临床试验显示达拉菲尼联合曲美替尼,再结合高剂量131I治疗对于恢复碘摄取有效。Ly等[10]开展一项COSMIC-311试验,研究卡博替尼群体药代动力学和暴露—反应分析,指导临床依据体表面积调整剂量更好地管理不良反应。Capdevila等[9]一项COSMIC-311试验发现无论患者的既往治疗史或病理类型如何,卡博替尼均能显著延长RAIR-DTC患者的无进展生存期。Matsuyama等[49]建议灵活调整仑伐替尼的药物间隔时间增加药物耐受性。Tateai等[50]发现高血压是导致仑伐替尼不耐受发生的主要原因,其次是手足皮肤反应和腹泻,建议进行规范的血压管理和临床护理。Mu等[51]研究发现初次碘治疗不摄碘的患者多伴随RAF、TERT启动子和TP53突变,而初次碘治疗摄碘患者多伴RET融合和RAS突变。上述文章均是关于探索新治疗方案中药物组合的协同效应、剂量优化、不良反应管控以及基于分子特征的精准医疗等相关研究。未来RAIR-DTC治疗领域会更倾向大样本量、多中心合作的高质量临床研究来探索新的治疗方案。此外,Redifferentiation近两年的突现也值得关注,随着对碘耐药分子机制NIS的深入,针对RAIR-DTC的更具靶向性的诱导再分化治疗手段已成为可能。目前针对MAPK通路的靶向药物主要有BRAF抑制剂和MEK抑制剂。BRAF抑制剂主要有达拉非尼(Dabrafenib)和维莫非尼(Vemurafenib),两者通过竞争性结合BRAF蛋白的ATP位点抑制其激酶活性,从而阻断MAPK信号通路以抑制肿瘤细胞的生长[52]。达拉非尼和曲美替尼两者联合可全面抑制MAPK的上、下游通路[53],有效恢复患者摄碘能力[48,54]。MEK抑制剂主要有司美替尼(Selumetinib)和曲美替尼(Trametinib)。司美替尼作为变构非竞争性MEK1/2蛋白抑制剂,可诱导细胞增殖停滞和细胞凋亡,Ho等发现其对RAS突变型的患者疗效更好[55]。曲美替尼是一种高特异性的MEK可逆性抑制剂,但相对于BRAFV600E突变的RAIR-DTC患者,曲美替尼对恢复RAS突变的RAIR-DTC患者131I摄取的疗效并不显著[48]。目前Buparlisib(AN2025)[56]、依维莫司(Everolimus)[57]两个PI3K/AKT/mTOR信号通路激动剂尚处于实验阶段,具有一定的发展潜力。小鼠模型中发现间变性淋巴瘤激酶(Anaplastic lymphoma kinase, ALK)抑制剂克唑替尼(Crizotinib)和色瑞替尼(Ceritinib)可逆转甲状腺癌细胞的去分化,恢复RAI的摄取[58]。但是缺乏长期随访的大样本数据指导、诱导再分后重新进行RAI治疗保证有临床获益的吸收剂量阈值缺乏循证、与其他靶向药物疗效缺乏头对头实验致获益群体不明确等问题在未来仍是挑战[59-60]。综上,随着越来越多针对不同靶点的新药涌现,更多高水平的临床试验应用会增加,以汇总并评估大量数据,更全面地了解治疗的效果和安全性,为RAIR-DTC治疗提供更精确、可持续的解决方案。
本研究应用文献计量分析发现,当前研究更多集中在酪氨酸激酶抑制剂和疾病预后因素方面。通过高质量临床试验提供的可靠数据优化临床获益,实现个体化、精准化治疗管理将成为未来的研究趋势。但本研究中最近发表的高质量文章引用频率低,可能尚未受到关注;本研究仅将WOS核心合集数据库中的英文文献纳入分析范畴,可能导致其他语种和数据库的文献被遗漏。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:曾萧贤:数据收集、整理及统计学处理,论文撰写张 弘:提出主要研究目标,文章审查 -
表 1 发文量与被引频次排名前10的作者
Table 1 Top 10 authors in terms of publication volume and citation frequency
Ranking Authors Number of publications (articles) Authors Cited frequency (times) 1 Schlumberger M 25 Brose MS 681 2 Brose MS 22 Schlumberger M 681 3 Leboulleux S 22 Dutcus C 359 4 Elisei R 20 Cabanillas ME 340 5 Wirth LJ 16 Haugen BR 328 6 Tahara M 15 Xing M 324 7 Capdevila J 15 Sherman SI 232 8 Chen LB 15 Bieber KC 175 9 Lin Y 15 Ho AL 158 10 Cabanillas ME 14 Leboulleux S 150 表 2 被引次数排在前10的参考文献
Table 2 Top 10 references in terms of citations
Ranking Title of literature Journal First Author Publication Number of
citations
(times)1 Lenvatinib versus placebo in radioiodine-refractory thyroid cancer New England Journal of Medicine Schlumberger M 2015 340 2 Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomized, double-blind, phase 3 trial. Lancet Brose MS 2014 326 3 Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limitations of radioiodine therapy. Clinical Endocrinology Durante Dutcus C 2006 288 4 2015 American Thyroid Association ManagementGuidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and DifferentiatedThyroid Cancer. Thyroid Haugen BR 2016 274 5 Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. New England Journal of Medicine Ho AL 2013 149 6 Phase Ⅱ trial of sorafenib in advanced thyroid cancer. Clinical Gupt-Abramson V 2008 105 7 Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic-differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Bieber KC 2010 104 8 Definition and management of radioactive iodine-refractory differentiated thyroid cancer Lancet Diabetes Endo Schlumberger M 2014 101 9 Integrated genomic characterization of papillarythyroid carcinoma Cell Agrawal N 2014 99 10 New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1) European Journal of Cancer Eisenhauer E 2009 99 -
[1] Van Nostrand D. Radioiodine refractory differentiated thyroid cancer: time to update the classifications[J]. Thyroid, 2018, 28(9): 1083-1093. doi: 10.1089/thy.2018.0048
[2] Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy[J]. Clin Endocr Metab, 2006, 91(8): 2892-2899. doi: 10.1210/jc.2005-2838
[3] Hicks D, Wouters P, Waltman L, et al. Bibliometrics: The Leiden Manifesto for research metrics[J]. Nature, 2015, 520(7548): 429-431. doi: 10.1038/520429a
[4] Ma C, Su H, Li H. Global research trends on prostate diseases and erectile dysfunction: a bibliometric and visualized study[J]. Front Oncol, 2021, 10: 627891. doi: 10.3389/fonc.2020.627891
[5] Kiyota N, Tahara M, Robinson B, et al. Impact of baseline tumor burden on overall survival in patients with radioiodine-refractory differentiated thyroid cancer treated with lenvatinib in the select global phase 3 trial[J]. Cancer, 2022, 128(12): 2281-2287. doi: 10.1002/cncr.34181
[6] Cabanillas ME, Schlumberger M, Jarzab B, et al. A phase Ⅱ trial of lenvatinib (e7080) in advanced, progressive, radioiodine-refractory, differentiated thyroid cancer: a clinical outcomes and biomarker assessment[J]. Cancer, 2015, 121(16): 2749-2756. doi: 10.1002/cncr.29395
[7] Deandreis D, Maillard A, Zerdoud S, et al. RADTHYR: an open-label, single-arm, prospective multicenter phase Ⅱ trial of radium-223 for the treatment of bone metastases from radioactive iodine refractory differentiated thyroid cancer[J]. Eur J Nucl Med Mol Imaging, 2021, 48(10): 3238-3249. doi: 10.1007/s00259-021-05229-y
[8] Taylor MH, Leboulleux S, Panaseykin Y, et al. Health-related quality-of-life analyses from a multicenter, randomized, double-blind phase Ⅱ study of patients with differentiated thyroid cancer treated with lenvatinib 18 or 24 mg/day[J]. Cancer Medicine, 2023, 12(4): 4332-4342. doi: 10.1002/cam4.5308
[9] Capdevila J, Krajewska J, Hernando J, et al. Increased progression-free survival with cabozantinib versus placebo in patients with radioiodine-refractory differentiated thyroid cancer irrespective of prior vascular endothelial growth factor receptor-targeted therapy and tumor histology: a subgroup analysis of the COSMIC-311 study[J]. Thyroid, 2024, 34(3): 347-359. doi: 10.1089/thy.2023.0463
[10] Ly NS, Li J, Faggioni R, et al. Population pharmacokinetics and exposure–response analysis for the phase 3 cosmic-311 trial of cabozantinib for radioiodine-refractory differentiated thyroid cancer[J]. Clin Pharmacokinet, 2023, 62(4): 587-598. doi: 10.1007/s40262-023-01210-0
[11] Brose MS, Cabanillas ME, Cohen EE, et al. Vemurafenib in patients with brafv600e-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial[J]. Lancet Oncol, 2016, 17(9): 1272-1282. doi: 10.1016/S1470-2045(16)30166-8
[12] Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer[J]. N Engl J Med, 2015, 372(7): 621-630. doi: 10.1056/NEJMoa1406470
[13] Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial[J]. Lancet, 2014, 384(9940): 319-328. doi: 10.1016/S0140-6736(14)60421-9
[14] 郑捷, 杨兴耀, 李想. 基于CiteSpace的推荐系统研究可视化分析[J]. 科学技术与工程, 2021, 21(34): 14634-14643. [Zheng J, Yang XY, Li X. Visualization Analysis of Recommendation System Based on CiteSpace[J]. Ke Xue Ji Shu Yu Gong Cheng, 2021, 21(34): 14634-14643.] doi: 10.3969/j.issn.1671-1815.2021.34.024 Zheng J, Yang XY, Li X. Visualization Analysis of Recommendation System Based on CiteSpace[J]. Ke Xue Ji Shu Yu Gong Cheng, 2021, 21(34): 14634-14643. doi: 10.3969/j.issn.1671-1815.2021.34.024
[15] 陈悦. 引文空间分析原理与应用: CiteSpace实用指南[M]. 北京: 科学出版社, 2014. [Chen Y. Principles and Applications of Citation Space Analysis: A Practical Guide to CiteSpace [M]. Beijing: Science Press, 2014.] Chen Y. Principles and Applications of Citation Space Analysis: A Practical Guide to CiteSpace [M]. Beijing: Science Press, 2014.
[16] 张亚奇, 朱锡群, 樊倩妤, 等. 碘难治性分化型甲状腺癌诱导再分化治疗研究进展[J]. 肿瘤防治研究, 2022, 49(10): 1086-1092. [Zhang YQ, Zhu XQ, Fan QY, et al. Progress in Re-differentiating Therapy of Radioiodine-refractory Differentiated Thyroid Cancer[J]. Zhong Liu Fang Zhi Yan Jiu, 2022, 49(10): 1086-1092.] doi: 10.3971/j.issn.1000-8578.2022.22.0011 Zhang YQ, Zhu XQ, Fan QY, et al. Progress in Re-differentiating Therapy of Radioiodine-refractory Differentiated Thyroid Cancer[J]. Zhong Liu Fang Zhi Yan Jiu, 2022, 49(10): 1086-1092. doi: 10.3971/j.issn.1000-8578.2022.22.0011
[17] Fugazzola L, Elisei R, Fuhrer D, et al. 2019 european thyroid association guidelines for the treatment and follow-up of advanced radioiodine-refractory thyroid cancer[J]. Eur Thyroid J, 2019, 8(5): 227-245. doi: 10.1159/000502229
[18] Cheng L, Fu H, Jin Y, et al. Clinicopathological features predict outcomes in patients with radioiodine-refractory differentiated thyroid cancer treated with sorafenib: a real-world study[J]. Oncologist, 2020, 25(4): e668-e678.
[19] Schneider TC, Abdulrahman RM, Corssmit EP, et al. Long-term analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a phase ii trial[J]. Eur J Endocrinol, 2012, 167(5): 643-650. doi: 10.1530/EJE-12-0405
[20] Ferrara N, Gerber HP, Lecouter J. The biology of vegf and its receptors[J]. Nat Med, 2003, 9(6): 669-676. doi: 10.1038/nm0603-669
[21] Olsson AK, Dimberg A, Kreuger J, et al. VEGF receptor signalling-in control of vascular function[J]. Nat Rev Mol Cell Biol, 2006, 7(5): 359-371. doi: 10.1038/nrm1911
[22] Ling Y, Xiong X, Luo J, et al. The efficacy and safety in radioactive iodine refractory thyroid cancer patients treated with sorafenib[J]. Front Endocrinol, 2023, 14: 1200932. doi: 10.3389/fendo.2023.1200932
[23] Zheng X, Xu Z, Ji Q, et al. A randomized, phase iii study of lenvatinib in chinese patients with radioiodine-refractory differentiated thyroid cancer[J]. Clin Cancer Res, 2021, 27(20): 5502-5509. doi: 10.1158/1078-0432.CCR-21-0761
[24] Damásio IL, Figueiredo A, Maciel J, et al. Effectiveness and safety of lenvatinib in a series of advanced well-differentiated thyroid carcinomas from a single tertiary cancer center and literature review[J]. Minerva Endocrinol (Torino), 2024. Online Agead of Print.
[25] Worden F, Rajkovic-Hooley O, Reynolds N, et al. Real-world treatment patterns and clinical outcomes in patients with radioiodine-refractory differentiated thyroid cancer (rai-r dtc) treated with first line lenvatinib monotherapy in the united states[J]. Endocrine, 2023, 84(2): 663-669. doi: 10.1007/s12020-023-03638-7
[26] Yu J, Liu Z, Su Y, et al. Tyrosine kinase inhibitors for radioiodine refractory differentiated thyroid cancer: a systematic review and meta-analysis[J]. Clin Endocrinol(Oxf), 2024, 100(4): 379-388. doi: 10.1111/cen.15027
[27] Revilla G, Al Qtaish N, Caruana P, et al. Lenvatinib-loaded poly(lactic-co-glycolic acid) nanoparticles with epidermal growth factor receptor antibody conjugation as a preclinical approach to therapeutically improve thyroid cancer with aggressive behavior[J]. Biomolecules, 2023, 13(11): 1647. doi: 10.3390/biom13111647
[28] Mikoshiba T, Sekimizu M, Kono T, et al. Utility and optimal management of planned drug holidays during lenvatinib treatment in patients with unresectable differentiated thyroid cancer: a real-world multi-center study[J]. Endocrine, 2024, 85(2): 777-785. doi: 10.1007/s12020-024-03744-0
[29] Tahara M, Takami H, Ito Y, et al. A prospective cohort study exploring the effect of lenvatinib planned drug holidays in treatment of differentiated thyroid cancer[J]. Thyroid, 2024, 34(5): 566-574. doi: 10.1089/thy.2023.0553
[30] Shen H, Zhu R, Liu Y, et al. Radioiodine-refractory differentiated thyroid cancer: molecular mechanisms and therapeutic strategies for radioiodine resistance[J]. Drug Resist Updat, 2024, 72: 101013. doi: 10.1016/j.drup.2023.101013
[31] 中国临床肿瘤学会核医学专家委员会, 中国临床肿瘤学会甲状腺癌专家委员会, 中华医学会核医学分会, 等. 放射性碘难治性分化型甲状腺癌诊治管理指南(2024版)[J]. 中华核医学与分子影像杂志, 2024, 44(6): 359-372. [Chinese Society of Clinical Oncology Nuclear Medicine Expert Committee, Thyroid Cancer Expert Committee of the Chinese Society of Clinical Oncology, Chinese Society of Nuclear Medicine, et al. Management guidelines for radioactive iodine-refractory differentiated thyroid cancer (2024 edition)[J]. Zhonghua He Yi Xue Yu Fen Zi Ying Xiang Za Zhi, 2024, 44(6): 359-372.] doi: 10.3760/cma.j.cn321828-20240125-00034 Chinese Society of Clinical Oncology Nuclear Medicine Expert Committee, Thyroid Cancer Expert Committee of the Chinese Society of Clinical Oncology, Chinese Society of Nuclear Medicine, et al. Management guidelines for radioactive iodine-refractory differentiated thyroid cancer (2024 edition)[J]. Zhonghua He Yi Xue Yu Fen Zi Ying Xiang Za Zhi, 2024, 44(6): 359-372. doi: 10.3760/cma.j.cn321828-20240125-00034
[32] Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo[J]. Cancer Sci, 2011, 102(7): 1374-1380. doi: 10.1111/j.1349-7006.2011.01939.x
[33] Lin Y, Qin S, Li Z, et al. Apatinib vs placebo in patients with locally advanced or metastatic, radioactive iodine–refractory differentiated thyroid cancer: the reality randomized clinical trial[J]. JAMA Oncol, 2022, 8(2): 242-250. doi: 10.1001/jamaoncol.2021.6268
[34] Lee YA, Lee H, Im SW, et al. NTRK and ret fusion–directed therapy in pediatric thyroid cancer yields a tumor response and radioiodine uptake[J]. J Clin Invest, 2021, 131(18): e144847. doi: 10.1172/JCI144847
[35] Elisei R, Grande E, Kreissl MC, et al. Current perspectives on the management of patients with advanced RET-driven thyroid cancer in Europe[J]. Front Oncol, 2023, 13: 1141314. doi: 10.3389/fonc.2023.1141314
[36] Haddad R, Elisei R, Hoff AO, et al. Diagnosis and Management of Tropomyosin Receptor Kinase Fusion-Positive Thyroid Carcinomas: A Review[J]. JAMA Oncol, 2023, 9(8): 1132-1141. doi: 10.1001/jamaoncol.2023.1379
[37] Sun D, Zhang X, Sun Y, et al. Early structural, biochemical, and metabolic responses to anlotinib in patients with progressive radioactive iodine refractory differentiated thyroid cancer[J]. Endocr Pract, 2024, 30(5): 456-464. doi: 10.1016/j.eprac.2024.02.005
[38] Dunn LA, Sherman EJ, Baxi SS, et al. Vemurafenib Redifferentiation of BRAF Mutant, RAI-Refractory Thyroid Cancers[J]. J Clin Endocrinol Metab, 2019, 104(5): 1417-1428. doi: 10.1210/jc.2018-01478
[39] Sousa Santos F, Joana Santos R, Leite V. Sorafenib and sunitinib for the treatment of metastatic thyroid cancer of follicular origin: a 7-year single-centre experience[J]. Eur Thyroid J, 2019, 8(5): 262-267. doi: 10.1159/000501680
[40] Chen J, Ji Q, Bai C, et al. Surufatinib in chinese patients with locally advanced or metastatic differentiated thyroid cancer and medullary thyroid cancer: a multicenter, open-label, phase Ⅱ trial[J]. Thyroid, 2020, 30(9): 1245-1253. doi: 10.1089/thy.2019.0453
[41] Nervo A, Retta F, Ragni A, et al. Management of progressive radioiodine-refractory thyroid carcinoma: current perspective[J]. Cancer Manag Res, 2022, 14: 3047-3062. doi: 10.2147/CMAR.S340967
[42] Kim CA, Kim M, Jin M, et al. Prognostic roles of inflammatory biomarkers in radioiodine-refractory thyroid cancer treated with lenvatinib[J]. Endocrinol Metab(Seoul), 2024, 39(2): 334-343. doi: 10.3803/EnM.2023.1854
[43] Sgrò D, Rossi P, Piaggi P, et al. Significance of Thyroglobulin Autoantibodies in Patients With Thyroid Cancer Treated With Lenvatinib[J]. J Endocr Soc, 2023, 7(8): bvad084. doi: 10.1210/jendso/bvad084
[44] Marotta V, Rocco D, Crocco A, et al. Survival predictors of radioiodine-refractory differentiated thyroid cancer treated with lenvatinib in real life[J]. J Clin Endocrinol Metab, 2024, 109(10): 2541-2552. doi: 10.1210/clinem/dgae181
[45] Majid O, Hayato S, Sreerama Reddy SH, et al. Population pharmacokinetic-pharmacodynamic modeling of serum biomarkers as predictors of tumor dynamics following lenvatinib treatment in patients with radioiodine-refractory differentiated thyroid cancer (rr-dtc)[J]. CPT Pharmacometrics Syst Pharmacol, 2024, 13(6): 954-969. doi: 10.1002/psp4.13130
[46] Takeuchi S, Hirata K, Magota K, et al. Early prediction of treatment outcome for lenvatinib using 18f-fdg pet/ct in patients with unresectable or advanced thyroid carcinoma refractory to radioiodine treatment: a prospective, multicentre, non-randomised study[J]. EJNMMI Res, 2023, 13(1): 69. doi: 10.1186/s13550-023-01019-9
[47] Gianoukakis AG, Choe JH, Bowles DW, et al. Real-world practice patterns and outcomes for rai-refractory differentiated thyroid cancer[J]. Eur Thyroid J, 2024, 13(1): e230039.
[48] Leboulleux S, Do Cao C, Zerdoud S, et al. A Phase Ⅱ Redifferentiation Trial with Dabrafenib-Trametinib and 131I in Metastatic Radioactive Iodine Refractory BRAF p. V600E-Mutated Differentiated Thyroid Cancer[J]. Clin Cancer Res, 2023, 29(13): 2401-2409. doi: 10.1158/1078-0432.CCR-23-0046
[49] Matsuyama C, Enokida T, Ueda Y, et al. Planned drug holidays during treatment with lenvatinib for radioiodine-refractory differentiated thyroid cancer: a retrospective study[J]. Front Oncol, 2023, 13: 1139659. doi: 10.3389/fonc.2023.1139659
[50] Tateai Y, Kawakami K, Teramae M, et al. Factors associated with lenvatinib adherence in thyroid cancer and hepatocellular carcinoma[J]. PLoS One, 2023, 18(11): e0294320. doi: 10.1371/journal.pone.0294320
[51] Mu Z, Zhang X, Sun D, et al. Characterizing Genetic Alterations Related to Radioiodine Avidity in Metastatic Thyroid Cancer[J]. Clin Endocr Metab, 2024, 109(5): 1231-1240. doi: 10.1210/clinem/dgad697
[52] Odogwu L, Mathieu L, Blumenthal G, et al. FDA approval summary: dabrafenib and trametinib for the treatment of metastatic non-small cell lung cancers harboring braf v600e mutations[J]. Oncologist, 2018, 23(6): 740-745. doi: 10.1634/theoncologist.2017-0642
[53] Nagarajah J, Le M, Knauf JA, et al. Sustained erk inhibition maximizes responses of brafv600e thyroid cancers to radioiodine[J]. J Clin Invest, 2016, 126(11): 4119-4124. doi: 10.1172/JCI89067
[54] Busaidy NL, Konda B, Wei L, et al. Dabrafenib versus dabrafenib + trametinib in braf -mutated radioactive iodine refractory differentiated thyroid cancer: results of a randomized, phase 2, open-label multicenter trial[J]. Thyroid, 2022, 32(10): 1184-1192.
[55] Ho AL, Dedecjus M, Wirth LJ, et al. Selumetinib plus adjuvant radioactive iodine in patients with high-risk differentiated thyroid cancer: A phase Ⅲ, randomized, placebo-controlled trial (Astra)[J]. J Clin Oncol, 2022, 40(17): 1870-1878. doi: 10.1200/JCO.21.00714
[56] Borson-Chazot F, Dantony E, Illouz F, et al. Effect of buparlisib, a pan-class i pi3k inhibitor, in refractory follicular and poorly differentiated thyroid cancer[J]. Thyroid, 2018, 28(9): 1174-1179. doi: 10.1089/thy.2017.0663
[57] Hanna GJ, Busaidy NL, Chau NG, et al. Genomic correlates of response to everolimus in aggressive radioiodine-refractory thyroid cancer: A phase Ⅱ study[J]. Clin Cancer Res, 2018, 24(7): 1546-1553. doi: 10.1158/1078-0432.CCR-17-2297
[58] Nikitski AV, Condello V, Divakaran SS, et al. Inhibition of alk-signaling overcomes strn-alk-induced downregulation of the sodium iodine symporter and restores radioiodine uptake in thyroid cells[J]. Thyroid, 2023, 33(4): 464-473. doi: 10.1089/thy.2022.0533
[59] Van Nostrand D, Veytsman I, Kulkarni K, et al. Redifferentiation of Differentiated Thyroid Cancer: Clinical Insights from a Narrative Review of Literature[J]. Thyroid, 2023, 33(6): 674-681. doi: 10.1089/thy.2022.0632
[60] 刘晶, 张俊. 放射性碘难治性分化型甲状腺癌再分化治疗研究进展[J]. 国际肿瘤学杂志, 2024, 51(7): 464-467. [Liu J, Zhang J. Progress in the study of redifferentiation therapy for radioactive iodine-refractory differentiated thyroid carcinoma[J]. Guo Ji Zhong Liu Xue Za Zhi, 2024, 51(7): 464-467.] doi: 10.3760/cma.j.cn371439-20231008-00076 Liu J, Zhang J. Progress in the study of redifferentiation therapy for radioactive iodine-refractory differentiated thyroid carcinoma[J]. Guo Ji Zhong Liu Xue Za Zhi, 2024, 51(7): 464-467. doi: 10.3760/cma.j.cn371439-20231008-00076



 下载:
下载: