Prognostic Significance of KMT2D Gene Mutation and Its Co-mutated Genes in Patients with Diffuse Large B-Cell Lymphoma
-
摘要:目的
探讨伴KMT2D基因突变弥漫性大B细胞淋巴瘤(DLBCL)患者的临床特征及其伴随突变基因对预后的影响。
方法选择155例初诊DLBCL患者资料,采用二代测序方法检测包括KMT2D突变在内的475种热点基因。根据有无KMT2D基因突变将患者分为KMT2D基因突变型组及KMT2D基因野生型组,比较两组患者临床特征及伴随突变基因的差异和生存差异。
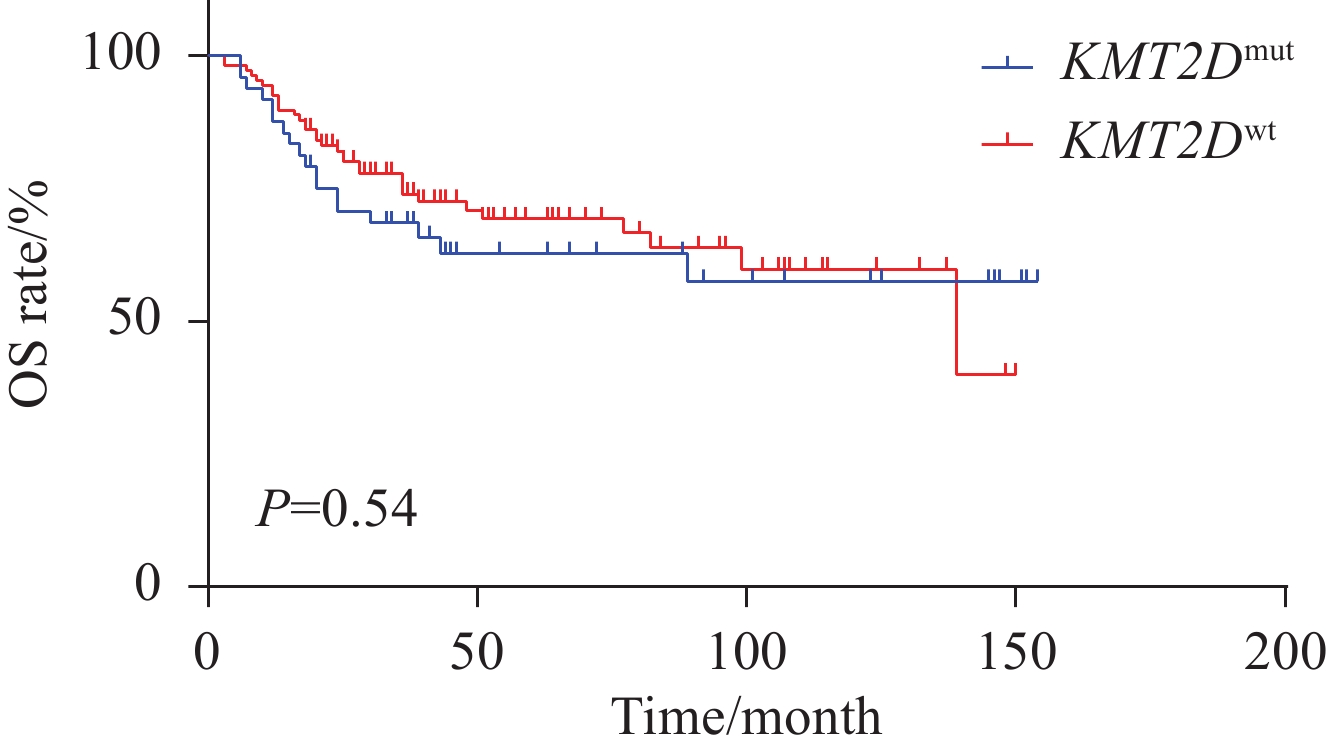
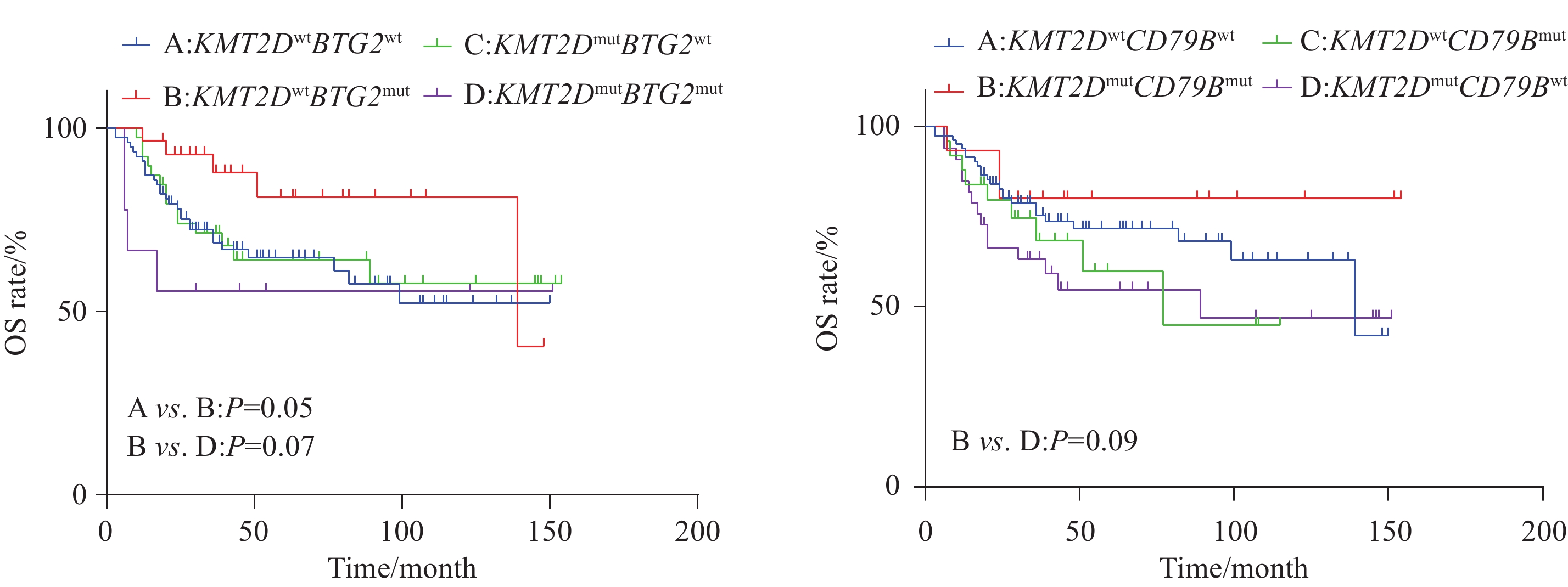
结果KMT2D突变频率为31%,突变型患者以老年居多(P=0.07),双表达阳性患者较少(P=0.07)。与KMT2D基因野生型相比,KMT2D基因突变分别与CDKN2A(OR=2.82,P=0.01)和BCL2(OR=3.84,P=0.016)基因共突变率高,而与MYC(OR=0.11,P=0.013)基因突变为相互排斥。单因素生存分析显示突变型组和野生型组总生存(OS)差异无统计学意义(P=0.54),KMT2DmutBTG2mut突变患者较KMT2Dwt BTG2mut(P=0.07)、KMT2Dwt BTG2wt(P=0.05)患者具有较差的OS,而 KMT2Dmut CD79Bmut 患者较KMT2Dmut CD79Bwt患者具有较好的OS趋势(P=0.09),未发现其他伴随基因对预后影响。多因素Cox回归分析结果显示,Ann Arbor分期为Ⅲ、Ⅳ期(HR=2.751,95%CI: 1.169-6.472,P=0.02)、LDH水平升高(HR=2.461,95%CI: 1.396-4.337,P=0.002)、Ki-67指数>80%(HR=1.875,95%CI: 1.066-3.299,P=0.029)及KMT2DmutBTG2mut(HR=4.566,95%CI: 1.348-15.471,P=0.015)是DLBCL患者OS的独立危险因素(P<0.05)。
结论KMT2D突变的DLBCL患者常伴随多种基因突变,其中伴BTG2基因突变患者预后较差。
-
关键词:
- 弥漫性大B细胞淋巴瘤 /
- 二代测序 /
- KMT2D基因 /
- 共突变 /
- 预后
Abstract:ObjectiveTo explore the clinical characteristics of patients with diffuse large B-cell lymphoma (DLBCL) accompanied with KMT2D gene mutation and the impact of its co-mutated genes on prognosis.
MethodsClinical data of 155 newly diagnosed DLBCL patients were obtained. The second-generation sequencing method was used to detect 475 hotspot genes, including KMT2D mutation. Patients were divided into the KMT2D mutation group and KMT2D wild-type group based on the presence or absence of KMT2D gene mutation. Clinical characteristics, differences in co-mutated genes, and survival differences between the two groups were compared.
ResultsThe frequency of KMT2D mutation was 31%, which is predominantly observed in elderly patients (P=0.07) and less in the double-expressor phenotype (P=0.07). Compared with the KMT2D wild-type group, KMT2D gene mutation was associated with higher co-mutation rates of CDKN2A (OR=2.82, P=0.01) and BCL2 (OR=3.84, P=0.016), while being mutually exclusive with MYC gene mutation (OR=0.11, P=0.013). In univariate survival analysis, no statistically significant difference in overall survival (OS) was found between the KMT2D mutation group and the wild-type group (P=0.54). Further analysis of the prognostic significance of KMT2D with other gene mutations indicated that patients with KMT2DmutBTG2mut had poorer OS than those with KMT2Dwt BTG2mut (P=0.07) and KMT2Dwt BTG2wt (P=0.05). On the contrary, patients with KMT2Dmut CD79Bmut had better OS than those with KMT2Dmut CD79Bwt (P=0.09), with no prognostic impact observed for other co-mutated genes. Multivariate Cox regression analysis revealed that Ann Arbor stages Ⅲ and Ⅳ (HR=2.751, 95%CI: 1.169-6.472, P=0.02), elevated LDH levels (HR=2.461, 95%CI: 1.396-4.337, P=0.002), Ki-67 index>80% (HR=1.875, 95%CI: 1.066-3.299, P=0.029), and KMT2DmutBTG2mut(HR=4.566, 95%CI: 1.348-15.471, P=0.015) were independent risk factors for OS in patients with DLBCL (P<0.05).
ConclusionDLBCL patients with KMT2D mutation often have multiple gene mutations, among which patients with a co-mutated BTG2 gene have poor prognosis.
-
Key words:
- Diffuse large B-cell lymphoma /
- Next-generation sequencing /
- KMT2D gene /
- Co-mutation /
- Prognosis
-
0 引言
弥漫性大B细胞淋巴瘤(Diffuse large B-cell lymphoma, DLBCL)是一种常见的非霍奇金淋巴瘤亚型。尽管免疫治疗对这类患者有显著疗效,但仍有约40%~50%的患者会出现复发或难治[1],主要原因是DLBCL在临床、免疫表型及分子遗传学等方面具有高度异质性[2]。研究表明,表观遗传改变与DLBCL的发生发展密切相关[3],其中,组蛋白甲基转移酶KMT2D基因是突变频率最高的表观遗传修饰基因。KMT2D(也称为MLL2/MLL4)编码 H3组蛋白 Lys-4位置的甲基化转移酶。研究发现,在DLBCL患者中KMT2D突变频率为24%~32%[4]。文献报道[5],与KMT2D野生型患者相比,KMT2D基因突变更多见于Ann Arbor期Ⅲ~Ⅳ和MYC表达高于40%的患者,Jiang等[6]将KMT2D的突变确定为淋巴瘤发生中的潜在早期驱动事件,并且导致DLBCL的复发。然而,随着二代测序技术在DLBCL领域的广泛应用,其他突变基因也陆续被发现,这些突变基因与KMT2D突变是否共同影响患者预后,仍需进一步探索。本研究分析155例DLBCL患者KMT2D基因突变情况,并探讨KMT2D突变与DLBCL患者的临床特征、伴发基因突变及与预后的关系,为利用KMT2D突变对DLBCL进行精准分层和预后判断提供更多的临床依据。
1 资料与方法
1.1 病例资料
回顾性分析2009年3月至2022年3月在新疆维吾尔自治区人民医院血液病科确诊、且留存石蜡包埋肿瘤组织标本的155例初诊DLBCL患者资料,DLBCL的诊断参照世界卫生组织淋巴肿瘤分类诊断标准《中国弥漫性大B细胞淋巴瘤诊断与治疗指南(2013年版)》[7],排除了入组前接受放、化疗的患者,合并有其他血液系统肿瘤及恶性消耗性疾病患者。采用二代测序技术对福尔马林固定石蜡包埋的肿瘤组织样本进行分析。收集患者年龄、性别、乳酸脱氢酶(LDH)、结外受累数目、病理组织学、免疫组织化学、影像学及骨髓检查等资料,分期采用Ann Arbor分期标准,预后评估采用国际预后指数(IPI)评分,细胞起源(COO)分型采用Hans分型方法。本研究在实施前获得本院医学伦理委员会批准(KY2022090201)。本研究性质属于回顾性研究,故患者无需签署知情同意书。
1.2 NGS测序
从患者诊断时的组织切片(经过福尔马林固定和石蜡包埋处理,简称FFPE)中提取基因组DNA。南京世和基因生物技术股份有限公司采用了安捷伦公司的SureSelect Human All Exon Kit(产自美国Santa Clara公司)进行靶向捕获测序。基因面板覆盖了475个与淋巴瘤相关的基因。本研究将基因突变频率超过10%的定义为高频突变基因,经筛选,共确定了40个高频突变基因。这些基因在Illumina Hi Seq平台上进行文库制备和测序,20×靶区覆盖率>90%,使用SNV/small indel完成检测、注释及统计。
1.3 随访
采用门诊、电话方式对所有患者进行随访,随访至2022年10月。总生存期(OS)定义为从入组开始至因为任何原因引起的死亡时间或随访截止时间;生存期以月为单位。
1.4 统计学方法
采用SPSS26.0软件对数据进行统计分析,对于两组间的分类变量,采用χ2检验或Fisher精确检验进行比较。生存分析采用Kaplan-Meier方法,并行Log-rank检验。多因素预后分析采用Cox比例风险回归模型。P<0.05为差异有统计学意义。
2 结果
2.1 临床基线资料
本研究155例DLBCL患者中KMT2D突变型患者48人,KMT2D野生型患者107人,临床特征见表1。在本队列中,KMT2D突变更多见于老年患者(P=0.07),而更少见于双表达阳性患者(P=0.07),但差异均无统计学意义。此外两组间在性别、细胞起源、Ann Arbor分期、LDH 水平、IPI评分、Ki-67表达量≥80%、结外受累的分布无显著差异(P>0.05)。
表 1 KMT2Dmut与KMT2Dwt患者的临床特征比较 (n(%))Table 1 Comparison of clinical characteristics between patients with KMT2Dmut and KMT2Dwt (n(%))Characteristics KMT2Dwt KMT2Dmut P Age(years) 0.07 >60 52(33.5) 31(20.0) ≤60 55(35.5) 17(11.0) Gender 0.85 Female 53(34.2) 23(14.8) Male 54(34.8) 25(16.1) Cell of origin 0.67 GCB 54(34.8) 26(16.8) Non-GCB 53(34.2) 22(14.2) Ann Arbor stage 0.20 Ⅲ/Ⅳ 79(51.0) 40(25.8) Ⅰ/Ⅱ 28(18.1) 8(5.2) LDH level 0.84 Elevated 42(27.1) 18(11.6) Normal 65(41.9) 30(19.4) IPI score 0.10 ≥3 54(34.8) 31(20.0) <3 53(34.2) 17(11.0) MYC/BCL2 double expression 0.07 Negative 73(58.4) 33(26.4) Positive 17(13.6) 2(1.6) Ki-67 expression 0.55 <80% 41(26.5) 16(10.3) ≥80% 66(42.6) 32(20.6) Number of extranodal sites 0.50 0-1 30(19.4) 11(7.1) ≥2 77(49.7) 37(23.9) Notes: LDH: lactate dehydrogenase; IPI: International Prognostic Index; GCB: germinal center B-cell. 2.2 KMT2D基因伴其他高频基因突变情况
48例KMT2D(31%)突变患者中,有45例(93.7%)伴其他高频基因突变,依次为PIM1(41.7%,20/48)、MYD88(35.4%,17/48)、CDKN2A(33.3%,16/48)、CD79B(31.2%,15/48)、FAT4(25%,12/48)、ETV6(25%,12/48)、BTG1(22.9%,11/48)、TP53(22.9%,11/48)、SOCS1(20.8%,10/48)、CREBBP(20.8%,10/48),此外BTG2、HIST1H1E、B2M、NFKBIE 21、BCL2共突变率均为18.7%(9/48),余发生伴随突变基因频率见图1。与KMT2D基因野生型相比,KMT2D基因突变分别与CDKN2A(OR=2.82, P=0.01)和BCL2(OR=3.84, P=0.016)基因共突变率更高,而与MYC(OR=0.11, P=0.013)基因突变相互排斥,差异均有统计学意义(均P<0.05),见图1。
2.3 KMT2D伴其他高频基因对DLBCL患者预后的影响
比较KMT2D突变型与野生型两组之间OS差异,结果显示,组间差异无统计学意义(P=0.54),见图2。进一步分析KMT2D伴其他基因突变是否对DLBCL患者预后产生影响。选择KMT2D及伴随基因共突变频率大于5%的基因进行分析,结果表明,仅KMT2D伴随BTG2、CD79B亚组分析具有预后预测价值,而其他伴随基因未发现对预后的影响。具体而言,在亚组分析中,根据KMT2D分别与BTG2、CD79B基因的伴随突变情况将患者分为4组进行生存分析。结果显示,KMT2DmutBTG2mut 突变患者较KMT2Dwt BTG2mut(P=0.07)、KMT2Dwt BTG2wt(P=0.05)患者具有较差的OS,而KMT2Dmut CD79Bmut 患者较KMT2Dmut CD79Bwt(P=0.09)患者具有较好的OS趋势,但差异无统计学意义,见图3。
2.4 KMT2D伴其他基因对DLBCL患者多因素分析
基于临床特征、KMT2D及伴随基因,对155例DLBCL患者进行单因素生存分析,结果见表2,Ann Arbor分期、LDH水平、IPI评分可影响DLBCL患者的预后。前面已分析KMT2DmutBTG2mut 及KMT2Dmut CD79Bmut对DLBCL患者预后有影响,为明确KMT2D伴有上述基因突变是否为影响DLBCL预后的危险因素,将单因素分析中P<0.1的预后指标纳入Cox多因素回归分析。因IPI评分包括Ann Arbor分期、LDH水平等多项指标,因此,本研究将P<0.1的IPI各项临床指标纳入多因素分析,结果显示,Ann Arbor分期为Ⅲ、Ⅳ(HR=2.751,95%CI: 1.169~6.472,P=0.02)、LDH水平升高(HR=2.461,95%CI: 1.396~4.337,P=0.002)Ki-67指数≥80%(HR=1.875,95%CI: 1.066~3.299,P=0.029)及KMT2DmutBTG2mut (HR=4.566,95%CI: 1.348~15.471,P=0.015)是DLBCL患者OS的独立危险因素(P<0.05)。
表 2 影响DLBCL患者OS的单因素生存分析Table 2 Univariate analysis of factors affecting OS of patients with DLBCLCharacteristics Univariate analysis(OS)
HR(95%CI)P Age 1.480(0.842-2.601) 0.173 Gender 0.711(0.409-1.235) 0.226 Cell of origin 0.734(0.421-1.278) 0.274 MYC/BCL2 double expression 0.653(0.269-1.588) 0.348 Number of extranodal sites 1.290(0.705-2.361) 0.408 Ann Arbor stage 2.572(1.095-6.038) 0.030 LDH level 2.308(1.323-4.025) 0.003 IPI score 2.539(1.371-4.704) 0.003 Ki-67 expression 0.625(0.360-1.084) 0.094 3 讨论
DLBCL是一种高度异质性疾病,NGS技术检测突变基因并进行危险度分层是实现精准治疗及改善预后的重要手段。随着NGS技术在临床上的广泛应用,越来越多的表观遗传调控相关基因突变被发现。其中,KMT2D是DLBCL中最常见的表观遗传调控基因,编码与B细胞分化和类别转换相关的组蛋白甲基转移酶。在DLBCL中,KMT2D基因的突变会降低其酶活性,进而导致生发中心B淋巴细胞的H3K4甲基化水平降低。在B细胞发育的早期阶段,KMT2D基因的条件性缺失会破坏B细胞分化的正常生理过程,使生发中心B细胞恶性生长[8]。自从Lohr和Pasqualucci等[9-10]利用全基因组外显子测序方法发现KMT2D基因在DLBCL中具有高突变率以来,对KMT2D基因与DLBCL之间关系的研究不断深入。本研究回顾性分析了155例DLBCL患者资料,结果显示,KMT2D基因突变的发生率为31%,与以往研究[9-10]结果一致。既往研究表明,KMT2D与DLBCL患者的临床特征存在关联[11],本研究KMT2D基因突变在老年患者中更为常见,提示KMT2D突变的发生可能与患者高龄有关,既往研究[11]同样证实了KMT2D与DLBCL患者年龄相关。此外,本研究中KMT2D基因在双表达阳性患者中突变频率较低,这与Chen等[12]研究结果有所不同:该研究表明,在双表达阳性组中,KMT2D基因突变频率更高。推测这种差异可能与本研究样本量较少、地域差异有关。今后仍需进一步扩大样本量进行深入探讨。
本研究KMT2D突变患者多存在其他基因伴随突变,且与野生型相比,KMT2D突变易与CDKN2A及BCL2突变共存,差异有统计学意义。目前尚未见到关于KMT2D与CDKN2A之间相关研究,而有研究报道[13],生发中心B细胞发生KMT2D的缺失并不可以驱动其发生恶性转化,但若合并Bcl-2异常则可促使恶性转化的发生。然而两者共突变是否在 DLBCL中也会发挥类似的协同作用值得深入研究。Reddy等[14]报道,KMT2D与MYC突变很少同时发生,这在本研究中也得到证实,提示两者之间存在互斥现象。然而KMT2D基因突变与其他基因突变的协同和互斥关系可能在DLBCL的发生、发展和演变过程中发挥作用的具体机制仍待进一步明确。
KMT2D突变与许多癌症的不良预后有关,例如非小细胞肺癌[15]、乳腺癌[16]和结直肠癌的卵巢转移[17];相反,KMT2D突变与小细胞肺癌[18]、食管鳞状细胞癌患者的生存期延长有关[19],表明KMT2D在肿瘤中的分子机制是复杂的。Rushton等[20]等对1 670例未经治疗的DLBCL患者中进行生存分析发现,KMT2D突变与较差的无进展生存期和总生存期相关,并且KMT2D截断突变与较差的无进展生存期相关。而Ortega-Molina等[8]对347例新诊断DLBCL患者生存分析发现,KMT2D突变与DLBCL患者总生存期、无进展生存期、疾病特异性生存期和进展时间无显著相关性。本研究KMT2D野生型和突变型患者生存差异无统计学意义,然而单个KMT2D突变对DLBCL的预后价值有限,因此,本研究进一步探索KMT2D联合其他基因突变在DLBCL患者中的预后意义。结果显示,KMT2DmutBTG2mut 患者的OS较KMT2DwtBTG2mut 患者具有较差趋势,并且KMT2DwtBTG2mut 患者的OS优于KMT2DwtBTG2wt 患者,提示在BTG2突变情况下,伴有KMT2D突变时导致DLBCL的OS预后更差,并且多因素分析显示KMT2DmutBTG2mut是DLBCL患者OS的独立危险因素。BTG2作为BTG/TOB基因家族第一个被发现的基因,已被证实在淋巴恶性肿瘤和实体瘤中发挥肿瘤抑制因子的作用[21] 。之前多项全基因组分析结果研究发现在B细胞恶性肿瘤中经常观察到BTG2的遗传畸变[9],表明BTG2突变可能减弱其肿瘤抑制因子作用从而导致肿瘤的发生发展。提示KMT2DmutBTG2mut可能作为DLBCL患者预后差的标志。
综上所述,KMT2D突变在DLBCL中常见,KMT2D突变常伴随多种基因突变,KMT2D及BTG2共突变是DLBCL患者OS的独立危险因素,这为基因突变对DLBCL进行精准分层和预后判断提供了更多的临床依据。鉴于本研究纳入病例数有限,结果存在一定局限性,未来可进行更大样本量的前瞻性研究进一步明确KMT2D突变及其伴随突变基因在DLBCL患者中的预后价值。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:木提拜尔·米吉提:收集整理数据、文献检索、统计分析、撰写文章漆小龙、热那古力·阿不来提、田文昕、刘沙、马卫媛、王增胜、安利、毛敏、木合拜尔·阿布都尔:收集数据、统计分析李 燕:收集数据、统计分析、审核文章 -
表 1 KMT2Dmut与KMT2Dwt患者的临床特征比较 (n(%))
Table 1 Comparison of clinical characteristics between patients with KMT2Dmut and KMT2Dwt (n(%))
Characteristics KMT2Dwt KMT2Dmut P Age(years) 0.07 >60 52(33.5) 31(20.0) ≤60 55(35.5) 17(11.0) Gender 0.85 Female 53(34.2) 23(14.8) Male 54(34.8) 25(16.1) Cell of origin 0.67 GCB 54(34.8) 26(16.8) Non-GCB 53(34.2) 22(14.2) Ann Arbor stage 0.20 Ⅲ/Ⅳ 79(51.0) 40(25.8) Ⅰ/Ⅱ 28(18.1) 8(5.2) LDH level 0.84 Elevated 42(27.1) 18(11.6) Normal 65(41.9) 30(19.4) IPI score 0.10 ≥3 54(34.8) 31(20.0) <3 53(34.2) 17(11.0) MYC/BCL2 double expression 0.07 Negative 73(58.4) 33(26.4) Positive 17(13.6) 2(1.6) Ki-67 expression 0.55 <80% 41(26.5) 16(10.3) ≥80% 66(42.6) 32(20.6) Number of extranodal sites 0.50 0-1 30(19.4) 11(7.1) ≥2 77(49.7) 37(23.9) Notes: LDH: lactate dehydrogenase; IPI: International Prognostic Index; GCB: germinal center B-cell. 表 2 影响DLBCL患者OS的单因素生存分析
Table 2 Univariate analysis of factors affecting OS of patients with DLBCL
Characteristics Univariate analysis(OS)
HR(95%CI)P Age 1.480(0.842-2.601) 0.173 Gender 0.711(0.409-1.235) 0.226 Cell of origin 0.734(0.421-1.278) 0.274 MYC/BCL2 double expression 0.653(0.269-1.588) 0.348 Number of extranodal sites 1.290(0.705-2.361) 0.408 Ann Arbor stage 2.572(1.095-6.038) 0.030 LDH level 2.308(1.323-4.025) 0.003 IPI score 2.539(1.371-4.704) 0.003 Ki-67 expression 0.625(0.360-1.084) 0.094 -
[1] Solimando AG, Annese T, Tamma R, et al. New insights into diffuse large B-cell lymphoma pathobiology[J]. Cancers (Basel), 2020, 12(7): 1869. doi: 10.3390/cancers12071869
[2] Koh Y. Genomics of diffuse large B cell lymphoma[J]. Blood Res, 2021, 56(S1): S75-S79. doi: 10.5045/br.2021.2021049
[3] Morin RD, Mendez-Lago M, Mungall AJ, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma[J]. Nature, 2011, 476(7360): 298-303. doi: 10.1038/nature10351
[4] Green MR. Chromatin modifying gene mutations in follicular lymphoma[J]. Blood, 2018, 131(6): 595-604. doi: 10.1182/blood-2017-08-737361
[5] Huang YH, Cai K, Xu PP, et al. CREBBP/EP300 mutations promoted tumor progression in diffuse large B-cell lymphoma through altering tumor-associated macrophage polarization via FBXW7-NOTCH-CCL2/CSF1 axis[J]. Signal Transduct Targeted Ther, 2021, 6(1): 10. doi: 10.1038/s41392-020-00437-8
[6] Jiang Y, Redmond D, Nie K, et al. Deep sequencing reveals clonal evolution patterns and mutation events associated with relapse in B-cell lymphomas[J]. Genome Biol, 2014, 15(8): 432.
[7] 中华医学会血液学分会, 中国抗癌协会淋巴瘤专业委员会. 中国弥漫大B细胞淋巴瘤诊断与治疗指南(2013年版)[J]. 中华血液学杂志, 2013, 34(9): 816-819. [Chinese Society of Hematology, Chinese Medical Association, and Chinese Society of Lymphoma, Chinese Anti-cancer Association. Chinese guidelines for diagnosis and treatment of diffuse large B cell lymphoma (2013)[J]. Zhonghua Xue Ye Xue Za Zhi, 2013, 34(9): 816-819.] Chinese Society of Hematology, Chinese Medical Association, and Chinese Society of Lymphoma, Chinese Anti-cancer Association. Chinese guidelines for diagnosis and treatment of diffuse large B cell lymphoma (2013)[J]. Zhonghua Xue Ye Xue Za Zhi, 2013, 34(9): 816-819.
[8] Ortega-Molina A, Boss IW, Canela A, et al. The histone lysine methyltransferase KMT2D sustains a gene expression program that represses B cell lymphoma development[J]. Nat Med, 2015, 21(10): 1199-1208. doi: 10.1038/nm.3943
[9] Lohr JG, Stojanov P, Lawrence MS, et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing[J]. Proc Natl Acad Sci U S A, 2012, 109(10): 3879-3884. doi: 10.1073/pnas.1121343109
[10] Pasqualucci L, Trifonov V, Fabbri G, et al. Analysis of the coding genome of diffuse large B-cell lymphoma[J]. Nat Genet, 2011, 43(9): 830-837. doi: 10.1038/ng.892
[11] Ye H, Lu L, Ge B, et al. MLL2 protein is a prognostic marker for gastrointestinal diffuse large B-cell lymphoma[J]. Int J Clin Exp Pathol, 2015, 8(10): 13043-13050.
[12] Chen H, Qin Y, Liu P, et al. Genetic profiling of diffuse large B-cell lymphoma: a comparison between double-expressor lymphoma and non-double-expressor lymphoma[J]. Mol Diagn Ther, 2023, 27(1): 75-86. doi: 10.1007/s40291-022-00621-2
[13] Zhang J, Dominguez-Sola D, Hussein S, et al. Disruption of KMT2D perturbs germinal center B cell development and promotes lymphomagenesis[J]. Nat Med, 2015, 21(10): 1190-1198. doi: 10.1038/nm.3940
[14] Reddy A, Zhang J, Davis NS, et al. Genetic and functional drivers of diffuse large B cell lymphoma[J]. Cell, 2017, 171(2): 481-494. doi: 10.1016/j.cell.2017.09.027
[15] Ardeshir-Larijani F, Bhateja P, Lipka MB, et al. KMT2D mutation is associated with poor prognosis in non–small-cell lung cancer[J]. Clin Lung Cancer, 2018, 19(4): e489-e501. doi: 10.1016/j.cllc.2018.03.005
[16] Ganesh K, Shah RH, Vakiani E, et al. Clinical and genetic determinants of ovarian metastases from colorectal cancer[J]. Cancer, 2017, 123(7): 1134-1143. doi: 10.1002/cncr.30424
[17] Kim JH, Sharma A, Dhar SS, et al. UTX and MLL4 coordinately regulate transcriptional programs for cell proliferation and invasiveness in breast cancer cells[J]. Cancer Res, 2014, 74(6): 1705-1717. doi: 10.1158/0008-5472.CAN-13-1896
[18] Simbolo M, Mafficini A, Sikora KO, et al. Lung neuroendocrine tumours: deep sequencing of the four World Health Organization histotypes reveals chromatin-remodelling genes as major players and a prognostic role for TERT, RB1, MEN1 and KMT2D[J]. J Pathol, 2017, 241(4): 488-500. doi: 10.1002/path.4853
[19] Zhang N, Shi J, Shi X, et al. Mutational characterization and potential prognostic biomarkers of chinese patients with esophageal squamous cell carcinoma[J]. Onco Targets Ther, 2020, 13: 12797-12809. doi: 10.2147/OTT.S275688
[20] Rushton CK, Arthur SE, Alcaide M, et al. Genetic and evolutionary patterns of treatment resistance in relapsed B-cell lymphoma[J]. Blood Adv, 2020, 4(13): 2886-2898. doi: 10.1182/bloodadvances.2020001696
[21] Kim SH, Jung IR, Hwang SS. Emerging role of antiproliferative protein BTG1 and BTG2[J]. BMB Rep, 2022, 55(8): 380-388. doi: 10.5483/BMBRep.2022.55.8.092



 下载:
下载: