-
摘要:目的
探讨局限期(LD)与广泛期(ED)原发性食管小细胞癌(PESCC)患者的临床特点及预后影响因素。
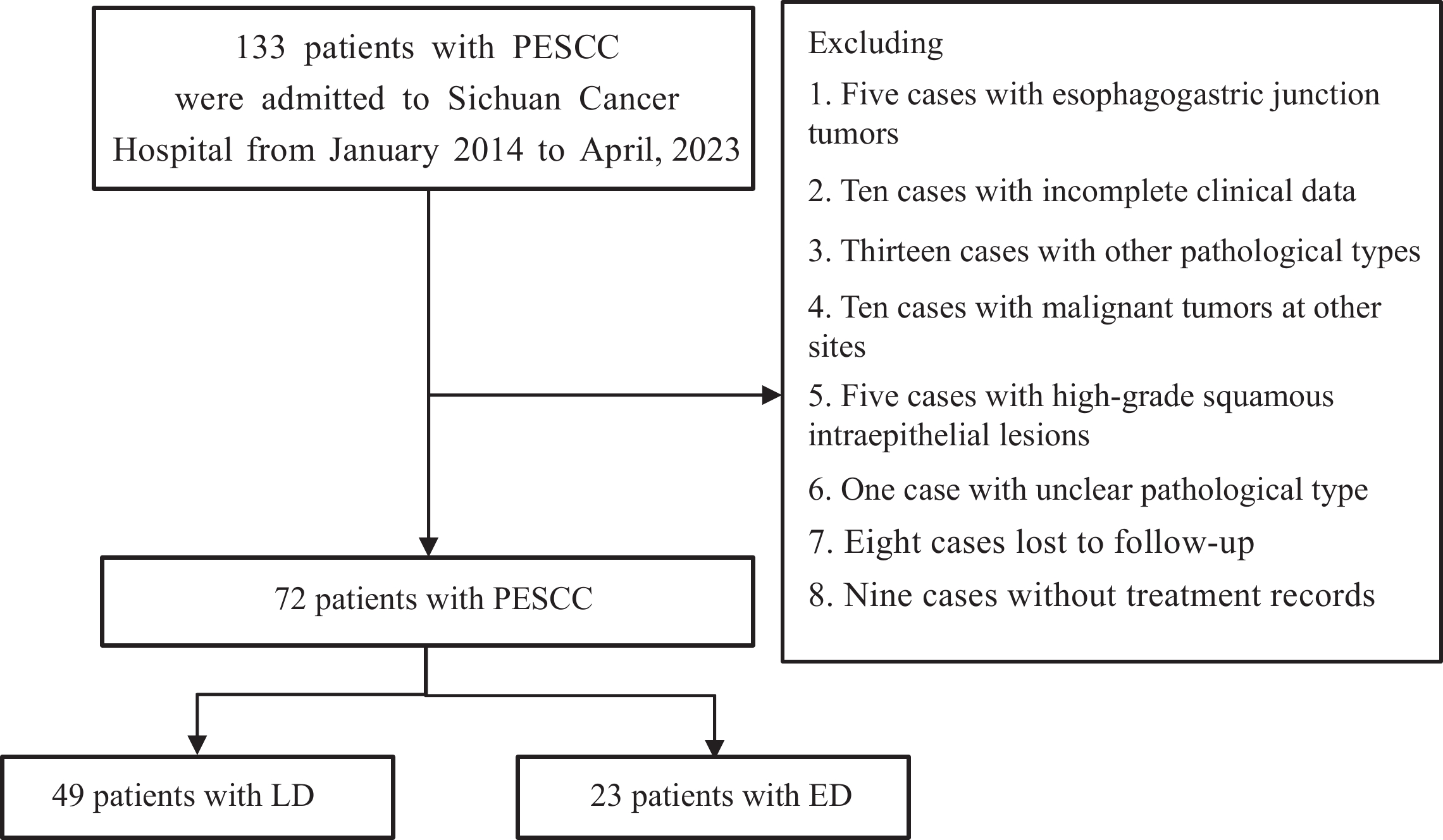
方法回顾性整理72例PESCC患者临床资料和随访信息。采用卡方检验比较LD与ED患者基线资料,Kaplan-Meier方法绘制二者生存曲线,组间生存率比较采用Log-rank检验。单因素和多因素Cox回归方法分析影响LD与ED患者总生存期的因素。
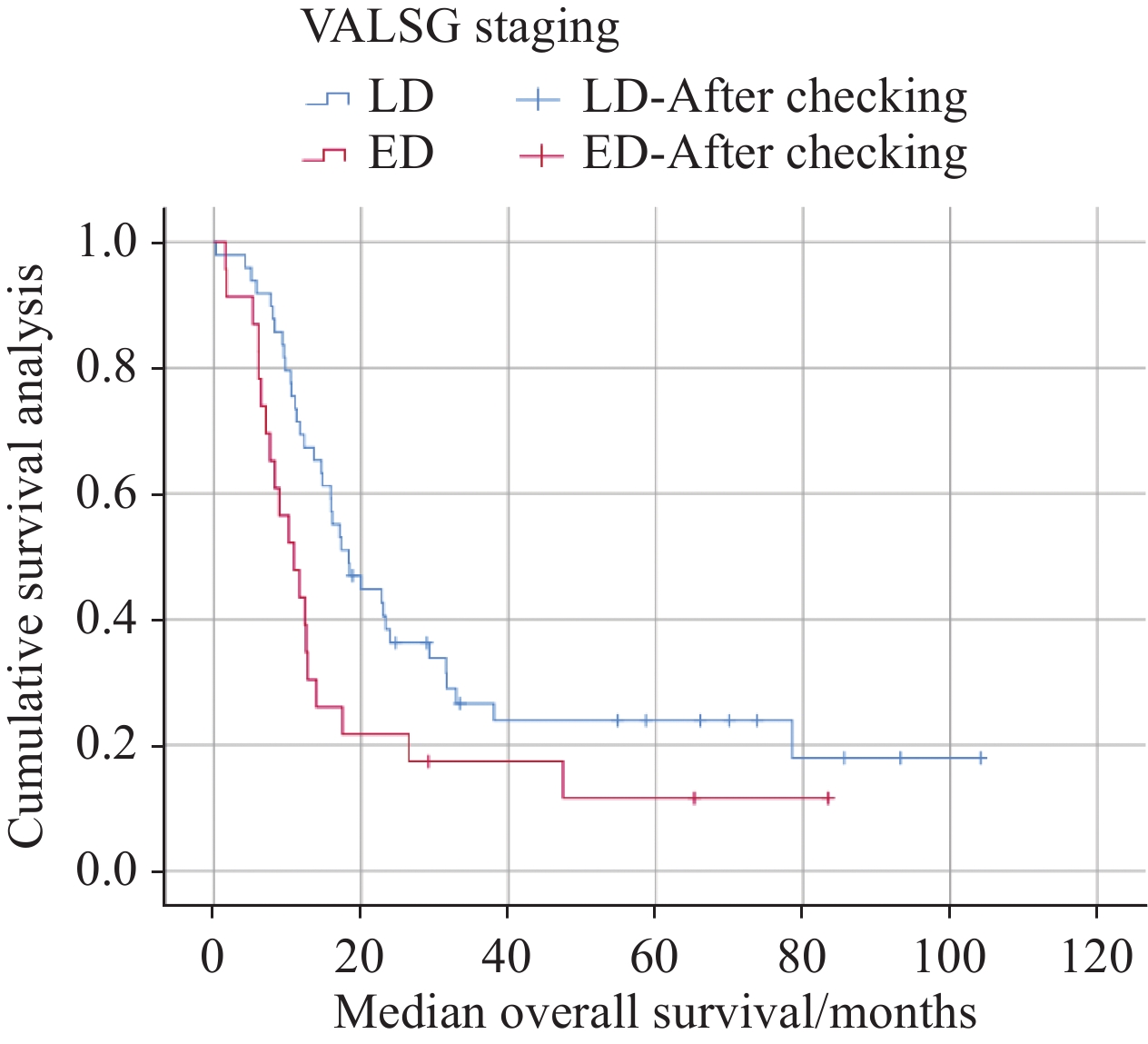
结果49例LD患者和23例ED患者纳入本研究。LD与ED患者的中位生存期分别为18.300月与10.903月(P=0.029)。总蛋白值(TP)(HR=0.890, 95%CI: 0.805~0.983, P=0.022)及化疗周期数(HR=0.388, 95%CI: 0.187~0.807, P=0.011)是LD患者预后独立影响因素;全身免疫炎症指数(SII)(HR=1.002, 95%CI: 1.000~1.004, P=0.007)与C反应蛋白值(CRP)(HR=1.065, 95%CI: 1.021~1.111, P=0.004)是ED患者预后独立影响因素。
结论PESCC恶性程度高,预后差。LD与ED的患者预后影响因素不同。总蛋白值及化疗周期是LD PESCC患者预后独立影响因素;全身免疫炎症指数及C反应蛋白值是ED PESCC患者预后独立影响因素。
Abstract:ObjectiveTo investigate the clinical characteristics and prognostic factors of the limited-stage disease (LD) and extensive-stage disease (ED) of primary esophageal small cell carcinoma (PESCC).
MethodsThe clinical data and follow-up information of 72 patients with PESCC were retrospectively analyzed. Chi-square test was used to compare the baseline data of patients with LD and ED, Kaplan-Meier method was employed to draw the survival curve of both groups, and Log-rank test was employed to compare survival rates between groups. Univariate and multivariate Cox regression methods were used to analyze the factors affecting the overall survival (OS) of patients with LD and ED.
ResultsA total of 49 patients with LD and 23 patients with ED were included in this study. The median survival time of patients with LD was 18.300 months and that of patients with ED was 10.903 months (P=0.029). Total protein (TP) value (HR=0.890, 95%CI: 0.805−0.983, P=0.022) and chemotherapy cycle number (HR=0.388, 95%CI: 0.187−0.807, P=0.011) were independent prognostic factors of patients with LD. Systemic immune-inflammation index (SII) (HR=1.002, 95%CI: 1.000−1.004, P=0.007) and C-reactive protein (CRP) (HR=1.065, 95%CI: 1.021−1.111, P=0.004) were independent prognostic factors of patients with ED.
ConclusionThe malignant degree of PESCC is high, and prognosis is poor. Patients with LD and ED have different prognostic factors. Total protein value and chemotherapy cycle are independent prognostic factors of patients with LD. SII and CRP are independent prognostic factors of patients with ED.
-
Key words:
- Primary esophageal small cell carcinoma /
- Limited-disease /
- Extensive-disease /
- Prognosis
-
0 引言
胃癌是起源于胃黏膜上皮细胞病变的消化道恶性肿瘤,具有较高的发病率与死亡率[1]。基因突变、不良饮食结构、病菌感染及吸烟等因素均可诱发胃癌。与传统开放根治手术相比,腹腔镜胃癌根治术因具有创伤较小、术后恢复较快等特点,已成为临床治疗胃癌的主要外科手段,可有效提高患者生存质量,延长生存期,但仍有部分患者在术后出现复发,严重影响患者预后[2]。因此,积极探讨影响胃癌腹腔镜根治术后复发的相关因素,以指导临床制定合适的防控措施,对减少患者术后复发具有重要意义。近年来,有研究显示[3]有多种基因、细胞因子及化合物参与了肿瘤细胞的发生发展过程,其中肿瘤坏死因子受体相关因子4(tumor necrosis factor receptor associated factor 4, TRAF4)和核糖体S6蛋白激酶4(ribosomal s6 protein kinase 4, RSK4)在肿瘤细胞中存在异常表达。TRAF4属于TRAF家族类蛋白,可介导肿瘤细胞内信号传递,有研究指出[4]TRAF4在乳腺癌、结直肠癌等肿瘤组织中高表达。RSK4为核糖体S6蛋白激酶家族成员之一,参与丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)信号通路的转导,具有调控细胞生长、增殖、分化的作用,有研究指出[5]RSK4蛋白在结直肠癌细胞株中低表达,可能参与了结直肠癌的发生发展。以往研究大多是对TRAF4或RSK4蛋白在癌细胞中的作用及其与患者临床病理特征的关系进行分析,但关于TRAF4和RSK4蛋白表达是否与胃癌患者腹腔镜根治术后复发相关不甚明确。鉴于此,本研究探讨了胃癌组织TRAF4和RSK4蛋白的表达与患者腹腔镜根治术后复发的相关性,旨在为临床制定合适的防控措施提供参考依据。
1 资料与方法
1.1 一般资料
选取本院2016年1月~2019年1月收治的176例胃癌患者的临床资料,其中男105例,女71例;年龄39~76岁,平均(53.18±10.09)岁;胃癌病变部位:胃窦80例,贲门55例,幽门28例,其他部位13例;胃癌组织类型:乳头状腺癌69例,管状腺癌55例,黏液腺癌39例,其他组织类型13例;TNM分期:Ⅰ期28例,Ⅱ期85例,Ⅲ期63例;肿瘤分化程度:高分化48例,中分化63例,低分化65例。本研究经医院伦理委员会批准通过。
纳入标准:(1)经临床病理及胃镜检查确诊为胃癌;(2)初治,满足腹腔镜胃癌根治术适应证且均接受腹腔镜胃癌根治术治疗及淋巴结清扫术;(3)患者或家属知晓并签署知情同意书。排除标准:(1)既往接受过放疗、靶向治疗、化疗或胃癌根治术者;(2)严重精神疾病患者;(3)既往胃癌术后出现复发的患者;(4)严重心、肾衰竭或功能不全者;(5)合并患有其他恶性肿瘤者;(6)术前已出现胃癌远处转移者;(7)合并患有免疫系统或血液系统疾病者;(8)术后预计生存期 < 3个月。
1.2 方法
1.2.1 TRAF4和RSK4蛋白表达检测
纳入研究患者均采取腹腔镜胃癌根治术治疗,并于术中留取胃癌组织及癌旁组织(距肿瘤切缘外约3 cm)。将组织经10%甲醛固定,常规石蜡包埋制备成组织蜡块,使用免疫组织化学法测定TRAF4和RSK4蛋白表达。步骤如下:将胃癌及癌旁组织蜡块连续切片(4 μm)3张,常规脱蜡水化,然后进行抗原修复,再使用1%过氧化氢(H2O2)-甲醇消除内源性过氧化物酶,室温下静置10 min后PBS洗3次,5%血清白蛋白(BSA)孵育20 min,分别加入兔抗人TRAF4多克隆抗体及兔抗人RSK4单克隆抗体,4℃孵育过夜。次日37℃复温1 h,后PBS洗3次,分别加入山羊抗兔二抗抗体,室温孵育2 h,PBS洗3次后,二氨基联苯胺(DAB)进行显色,蒸馏水清洗终止显色,苏木精对比染色,充分水洗。脱水后,以中性树胶封固,于显微镜下观察。同时用PBS代替一抗作为阴性对照。
1.2.2 结果判定
在400高倍镜视野下随机选取5个视野,各视野均计数100个细胞,记录阳性细胞占比,其中TRAF4阳性细胞表现为细胞核或细胞质出现棕黄色或棕褐色颗粒,RSK4阳性细胞表现为细胞质出现棕黄色或棕褐色颗粒。
蛋白表达情况判定标准:(1)按照染色强度判定:细胞未着色记为0分,呈淡棕黄色记为1分,呈棕黄色记为2分,呈明显的棕黄色或深褐色记为3分;(2)按照阳性细胞(棕黄色)所占比例判定:阳性细胞占比 < 5%记为0分,5%≤阳性细胞占比 < 25%记为1分,25%≤阳性细胞占比 < 50%记为2分,50%≤阳性细胞占比 < 75%记为3分,阳性细胞占比≥75%记为4分;将染色强度与阳性细胞占比两者得分相乘, < 2分为蛋白表达阴性,≥2分为蛋白表达阳性。
术后复发判定与分组:腹腔镜胃癌根治术后,前3个月每月对患者电话或微信随访一次,上门访问一次,3个月后每隔1个月电话随访1次,随访期间嘱患者定期至医院复诊,共随访36个月。复发判定:经影像学检查(CT、PET-CT、MRI)、组织病理学检查(胃镜活检、穿刺或再次手术)明确,发现手术切除部位或附近新发肿瘤或出现远处组织器官转移等。将术后复发者归为复发组,反之归为未复发组。
1.2.3 影响因素分析
统计可能影响胃癌患者腹腔镜根治术后复发的影响因素,包括患者年龄、性别、最大肿瘤直径、分化程度、TNM分期、Lauren分型、浸润深度、腹水、淋巴结转移、术后辅助化疗、TRAF4蛋白表达、RSK4蛋白表达、规律饮食、家族胃癌病史、幽门螺旋杆菌感染等,将其作为自变量(X),对其进行赋值,结果见表 1;另将腹腔镜根治术后复发情况作为因变量(Y),未复发记为0,复发记为1;分析因变量与自变量间的关系。
表 1 胃癌患者腹腔镜根治术后复发影响因素赋值情况Table 1 Assignment of independent variables influencing recurrence after laparoscopic radical gastrectomy
1.3 观察指标
(1)胃癌组织与癌旁组织TRAF4和RSK4蛋白表达;(2)不同病理特征患者胃癌组织TRAF4和RSK4蛋白表达;(3)术后复发情况及复发组与未复发组胃癌组织TRAF4和RSK4蛋白表达比较;(4)胃癌患者腹腔镜根治术后复发的影响因素:风险比(HR)、95%CI;(5)胃癌组织TRAF4和RSK4蛋白表达对腹腔镜根治术后复发的预测作用。
1.4 统计学方法
使用SPSS23.0软件进行统计学分析,计数资料采用例数和百分数(n(%))表示,采用χ2检验。当理论频数为1~5则采用校正卡方检验,当理论频数 < 1或样本量 < 40则采用Fisher精确检验;等级比较采用秩和检验;采用单因素和多因素Cox回归分析法分析胃癌腹腔镜根治术后复发的影响因素;以术后实际复发情况为金标准,计算各影响因素预测术后复发的敏感度、特异性与准确度,比较采用χ2检验。P < 0.05为差异有统计学意义。
2 结果
2.1 胃癌组织与癌旁组织TRAF4和RSK4蛋白表达比较
胃癌组织中TRAF4蛋白阳性表达率高于癌旁组织(P < 0.05),RSK4蛋白阳性表达率低于癌旁组织(P < 0.05),见表 2、图 1。
表 2 胃癌组织与癌旁组织TRAF4和RSK4蛋白表达比较(n(%))Table 2 Comparison of TRAF4 and RSK4 protein expression between gastric cancer tissues and adjacent tissues (n(%))
2.2 不同病理特征患者胃癌组织TRAF4和RSK4蛋白表达比较
低分化、TNM Ⅲ期、浸润深度T3~T4、淋巴结转移患者胃癌组织TRAF4蛋白阳性表达率均分别高于中/高分化、TNMⅠ/Ⅱ期、浸润深度T1~T2、无淋巴结转移患者(P < 0.05),而RSK4蛋白阳性表达率分别低于中/高分化、TNMⅠ/Ⅱ期、浸润深度T1~T2、无淋巴结转移患者(P < 0.05),见表 3。
表 3 不同病理特征患者胃癌组织TRAF4和RSK4蛋白表达比较(n(%))Table 3 Comparison of TRAF4 and RSK4 protein expression in gastric cancer tissues of patients with different pathological characteristics (n(%))
2.3 复发组与未复发组胃癌组织一般资料比较
对176例行腹腔镜根治术的胃癌患者进行36个月的术后随访,随访率100%,其中有56例患者胃癌复发,120例患者未复发,复发率为31.82%(56/176)。复发组胃癌组织TRAF4蛋白阳性表达率高于未复发组(P < 0.05),RSK4蛋白阳性表达率低于未复发组(P < 0.05),复发组最大肿瘤直径、分化程度、TNM分期、浸润深度、淋巴结转移、术后辅助化疗、规律饮食、家族胃癌病史与未复发组比较,差异有统计学意义(P < 0.05),见表 4。
表 4 复发组与未复发组一般资料比较(n(%))Table 4 Comparison of general data between recurrence group and non-recurrence group (n(%))
2.4 影响腹腔镜胃癌根治术后复发的单因素Cox回归分析
结果显示最大肿瘤直径 > 5 cm、低分化程度、TNM分期Ⅲ期、浸润深度T3~T4、淋巴结转移、术后未辅助化疗、TRAF4蛋白阳性表达、家族胃癌病史均是腹腔镜胃癌根治术后患者复发的危险因素(P < 0.05),而RSK4蛋白阳性表达、规律饮食是保护因素(P < 0.05),见表 5。
表 5 影响腹腔镜胃癌根治术后复发的单因素Cox回归分析Table 5 Cox regression analysis of single factor influencing recurrence after laparoscopic radical gastrectomy
2.5 影响腹腔镜胃癌根治术后复发的多因素分析
以单因素Cox回归分析中P < 0.10的因素为自变量,进行多因素Cox回归分析,结果显示最大肿瘤直径 > 5 cm、低分化程度、TNM分期Ⅲ期、浸润深度T3~T4、淋巴结转移、术后未辅助化疗、TRAF4蛋白阳性表达均是胃癌患者腹腔镜根治术后复发的危险因素(P < 0.05),而RSK4蛋白阳性表达、规律饮食均是保护因素(P < 0.05),见表 6。
表 6 影响腹腔镜胃癌根治术后复发的多因素Cox回归分析Table 6 Cox regression analysis of multiple factors influencing recurrence after laparoscopic radical gastrectomy
2.6 TRAF4和RSK4蛋白表达对胃癌腹腔镜根治术后复发的预测作用
胃癌组织TRAF4和RSK4蛋白表达联合预测胃癌腹腔镜根治术后复发的准确度高于各蛋白单独预测(χ2=34.566、47.239, P < 0.001),且高于最大肿瘤直径 > 5 cm、低分化、TNMⅢ期、浸润深度T3~T4、淋巴结转移、术后未辅助化疗、未规律饮食(χ2=18.810、4.380、15.286、17.902、6.588、10.570、20.682,P=0.000、0.036、0.000、0.000、0.010、0.001、0.000),而与TRAF4、RSK4蛋白表达联合其他七项比较差异无统计学意义(χ2=2.982, P=0.084),见表 7。
表 7 TRAF4和RSK4蛋白表达对胃癌腹腔镜根治术后复发的预测作用Table 7 Predictive effect of TRAF4 and RSK4 protein expression on recurrence of gastric cancer after laparoscopic radical surgery
3 讨论
胃癌是临床常见的消化道恶性肿瘤之一,近年来,我国胃癌发病率逐渐上升且呈年轻化趋势,严重威胁患者的身心健康[6]。腹腔镜根治术是目前临床治疗胃癌较为广泛的微创术式,但术后复发仍是不容忽视的问题[7]。本研究中,胃癌患者腹腔镜根治术后3年的复发率为31.82%,稍低于赵车冬等[8]研究显示的胃癌根治术后复发率(术后随访3年,复发率41.67%),可能与所选样本间的个体差异性、具体手术方式、术后干预措施等差异有关,但均表明胃癌根治术后有较高的复发风险,严重影响患者预后。因此,对影响胃癌腹腔镜根治术后复发的因素进行探索分析很重要。
本研究显示,胃癌组织中TRAF4蛋白阳性表达率较癌旁组织高,RSK4蛋白阳性表达率较癌旁组织低,提示TRAF4、RSK4蛋白在胃癌组织中均异常表达,可能与胃癌的发生发展有关。TRAF家族蛋白主要参与细胞的增殖、分化及凋亡的调控,在多种生命活动中发挥重要的作用,其中TRAF4通过间接作用或形成复合体的方式在细胞质、细胞膜和细胞核中迁移而发挥作用[9]。TRAF4与丝裂原活化蛋白激酶激酶4结合可激活c-Jun氨基酸末端激酶(JNK)途径,而JNK途径与肿瘤发生密切相关[10]。有研究表明[11],结直肠癌组织中TRAF4蛋白阳性表达率显著高于癌旁组织,与本研究结果相似。RSK4作为细胞内重要的调控因子,可以调节细胞周期(G0/S期、G1/S期)细胞比例,对癌细胞的过度增殖具有抑制作用[12]。有研究显示[13]RSK4蛋白在多种肿瘤细胞中如乳腺癌、结直肠癌等低表达,表明RSK4蛋白可能具有类似抑癌基因的作用。因而胃癌组织中RSK4蛋白阳性表达率较癌旁组织低,且RSK4蛋白阳性表达率下降会降低机体对肿瘤发生的调控作用,增加肿瘤发生的风险。另本研究结果显示,低分化、TNMⅢ期、浸润深度T3~T4、淋巴结转移患者胃癌组织TRAF4蛋白阳性表达率较中/高分化、TNMⅠ/Ⅱ期、浸润深度T1~T2、无淋巴结转移患者高,而RSK4蛋白阳性表达率较中/高分化、TNMⅠ/Ⅱ期、浸润深度T1~T2、无淋巴结转移患者低,提示TRAF4和RSK4蛋白表达可能与胃癌患者分化程度、TNM分期、浸润深度以及淋巴结转移情况存在一定的关联。TRAF4可通过调控PI3K/AKT/Oct4、Wnt/β-catenin等多条信号通路促进癌细胞的增殖、侵袭、迁移以及上皮间质转化[14-15]。RSK4表达下降可促使其阻碍细胞蛋白合成的作用减弱,丧失保护肿瘤细胞分化调控作用,抑制肿瘤生长的功能减退,导致肿瘤进展,其过表达可能通过抑制PI3K/AKT信号通路而抑制肿瘤细胞迁移、侵袭与生长[16]。
本研究结果还显示,复发组胃癌组织TRAF4蛋白阳性表达率高于未复发组,RSK4蛋白阳性表达率低于未复发组,且多因素分析表明TRAF4蛋白阳性表达是胃癌患者腹腔镜根治术后复发的危险因素,RSK4蛋白阳性表达是其保护因素,提示胃癌组织TRAF4和RSK4蛋白表达情况与患者根治术后复发密切相关。TRAF4是一种定位于细胞核内的重要信号转导蛋白,已被证实参与了多种肿瘤细胞的发生发展过程。相关研究报道[17]显示,TRAF4表达上调可以诱导G1/S-特异性周期蛋白-D1、调节基因、癌基因、聚ADP核糖聚合酶1等靶分子的表达,还具有激活Wnt/β-catenin信号通路促进癌细胞生长、迁移的作用。因此,TRAF4蛋白高表达可促进肿瘤进一步生长与转移,增加胃癌患者术后复发的风险。RSK4是位于细胞质内的一种核糖体修饰因子,可以通过对核糖体羧基末端进行修饰而降低染色体DNA配对的错配风险,还可以提高错配修复因子半胱氨酸的活性[18]。另RSK4对癌细胞低分化具有重要的抑制作用,可降低癌细胞黏附血管内皮或淋巴管内皮的能力,抑制癌症进展[19]。有研究表明[20],过表达RSK4能够抑制乳腺癌细胞的侵袭与转移。当RSK4蛋白表达降低时,其抑制癌细胞低分化、侵袭与转移等的能力减弱,则肿瘤进展的风险增加,患者术后复发的概率升高。因此,RSK4蛋白阳性表达可以及时监控肿瘤的发生,抑制癌细胞低分化,为胃癌患者腹腔镜根治术后复发的保护因素。本研究结果显示,TRAF4和RSK4蛋白表达联合用于预测胃癌腹腔镜根治术后复发中具有较高的准确度,提示临床可根据此两种蛋白表达情况而对胃癌患者根治术后复发进行评估,尽可能及早地给予针对性干预对策以减少术后复发。
此外,本研究结果显示,最大肿瘤直径 > 5 cm、低分化程度、TNMⅢ期、浸润深度T3~T4、淋巴结转移、术后未辅助化疗亦均是胃癌患者腹腔镜根治术后复发的危险因素,而规律饮食是其保护因素。淋巴管壁较薄且管径较细,常与静脉或血管伴行,当肿瘤直径越大,侵犯周围淋巴结的程度就越深,当淋巴管壁被肿瘤细胞逐步浸润后,进入淋巴管随淋巴液被带至淋巴结,并以此为中心长出同样肿瘤,最终引起肿瘤转移至淋巴结[21]。有研究显示[22],术前出现淋巴结转移的肿瘤患者术后复发率超50%,与本研究结果一致,表明淋巴结转移可能在肿瘤复发中起重要作用,成为术后评估肿瘤复发转移的重要预测指标;与此同时,肿瘤直径越大,浸润程度越深,就会越容易突破浆膜层对周围组织或肝、肾器官产生侵犯,可能会导致肿瘤发生转移,引起局部复发[23]。分化程度越低,TNM分期越高,表示恶性程度越高,出现淋巴结转移的可能性越大,术后复发的风险越高[24]。行根治性手术治疗时,可能存在微小或潜在癌灶没有发现而未能完全被清除,术后实施一定周期的辅助化疗可杀灭癌细胞,控制肿瘤进展,但若未进行化疗干预,残留的病灶则可逐渐发展甚或转移,导致术后复发。有研究表明[25],术后化疗 > 3周期的患者相较于未化疗或化疗≤3周期的患者具有更低的术后复发率。另规律饮食可以保证机体营养的正常供应,提高患者免疫力,降低术后复发的风险。
综上所述,TRAF4蛋白在胃癌组织异常高表达,而RSK4蛋白则异常低表达,二者异常表达均与胃癌患者腹腔镜根治术后复发密切相关,可对术后复发进行较好地预测。最大肿瘤直径 > 5 cm、低分化程度、TNMⅢ期、浸润深度T3~T4、淋巴结转移、术后化疗情况、是否规律饮食等是胃癌腹腔镜根治术后复发的影响因素,临床中可据此加强对胃癌患者的监督与管理,制定针对性干预方案,以促进患者预后改善。本研究通过对胃癌组织中TRAF4和RSK4蛋白表达情况与患者腹腔镜根治术后复发的关系分析,而为临床术后及时制定合理的防控方案提供了指导依据,同时亦为胃癌的治疗研究提供了一个新方向,但本研究纳入复发的样本量较小,且未对深层作用机制进行探讨,后续需扩大样本量进一步探索。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:全 敏:试验设计、数据收集、文章撰写唐嘉源:数据整理、文献检索陈 红:选题、研究方法设计、文章审阅 -
表 1 LD与ED患者基线资料差异对比
Table 1 Comparison of baseline data between LD and ED patients
Variables LD patients
(n = 49)ED patients
(n = 23)χ2 P Gender 0.421 0.421 Male 37 20 Female 12 3 Age(years) 2.500 0.114 <65 31 10 ≥65 18 13 Smoking history 0.533 0.465 Yes 32 17 No 17 6 Drinking history 0.659 0.417 Yes 27 15 No 22 8 Family history <0.001 0.992 Yes 8 3 No 41 20 BMI 0.827 0.363 <24 37 15 ≥24 12 8 First symptom 0.125 0.723 Dysphagia 34 15 Others 15 8 Notes: BMI: body mass index; Others: hoarse, pain, neck mass, epigastric discomfort. 表 2 PESCC患者一般连续性变量统计描述
Table 2 Statistical description of general continuity variables in patients with PESCC
Variables Mean value Standard deviation Age 64.39 7.734 BMI 22.74 3.234 Focal length 6.39 2.778 NEUT# 4.00 1.205 LYMPH# 1.59 0.657 PLT 193.74 61.699 Mono# 0.42 0.168 NLR 2.87 1.428 PLR 136.90 61.24 SII 557.75 372.882 PNI 45.744 9.366 CRP 7.24 13.497 TP 65.34 4.745 ALB 39.44 3.151 Notes: NEUT#: neutrophil count; LYMPH#: lymphocyte count; PLT: platelet count; MONO#: monocyte count; TP: total protein; ALB: serum albumin. 表 3 LD与ED患者总生存期的Cox单因素生存分析
Table 3 Cox univariate survival analysis of overall survival of patients with LD and ED
Variables LD patients ED patients HR 95%CI P HR 95%CI P Gender 1.160 0.527-2.551 0.713 0.840 0.240-2.935 0.785 Age 1.034 0.983-1.087 0.195 1.040 0.967-1.118 0.287 Smoking history 1.269 0.624-2.580 0.511 0.771 0.288-2.063 0.605 Drinking history 1.085 0.560-2.100 0.809 0.539 0.211-1.376 0.196 Family history 0.503 0.194-1.302 0.157 0.721 0.164-3.157 0.664 BMI 0.591 0.268-1.302 0.192 0.880 0.759-1.019 0.088 Focal length 1.039 0.927-1.165 0.509 0.954 0.830-1.097 0.507 NEUT# 0.899 0.663-1.219 0.494 1.469 0.875-2.464 0.146 LYMPH# 0.785 0.474-1.300 0.346 0.521 0.217-1.247 0.143 PLT 0.997 0.990-1.003 0.328 1.001 0.993-1.009 0.809 Mono# 0.871 0.129-5.897 0.888 1.115 0.040-30.957 0.949 NLR 1.016 0.815-1.266 0.887 1.819 1.216-2.719 0.004 PLR 1.001 0.995-1.006 0.853 1.011 1.003-1.019 0.009 SII 1.000 0.999-1.001 0.741 1.002 1.001-1.004 0.003 PNI 0.950 0.921-0.979 0.001 0.941 0.857-1.034 0.207 TP 0.915 0.849-0.987 0.022 1.029 0.926-1.142 0.597 ALB 0.937 0.847-1.037 0.209 0.942 0.794-1.119 0.497 CRP 1.020 0.974-1.068 0.395 1.059 1.009-1.112 0.021 Treatment mode* 0.934 0.490-1.782 0.836 - - - Treatment mode** 0.503 0.247-1.024 0.058 0.800 0.331-1.033 0.620 Chemotherapy cycle 0.454 0.230-0.897 0.023 1.324 0.538-3.260 0.542 Notes: *: surgical vs. nonsurgical treatment; **: combination therapy vs. monotherapy; -: not applicable. 表 4 影响LD与ED患者总生存期的Cox多因素生存分析
Table 4 Cox multifactor survival analysis of overall survival of patients with LD and ED
Variables B SE Wald DF P HR 95%CI LD TP −0.117 0.051 5.226 1 0.022 0.890 0.805-0.983 Chemotherapy cycle −0.946 0.373 6.427 1 0.011 0.388 0.187-0.807 ED SII 0.002 0.001 7.362 1 0.007 1.002 1.000-1.004 CRP 0.063 0.022 8.511 1 0.004 1.065 1.021-1.111 -
[1] 蔡高科, 陈永顺. 原发性食管小细胞癌的治疗进展[J]. 微循环学杂志, 2022, 32(4): 75-79, 84. [Cai GK, Chen YS. The Research Progress of Treatment on Primary Small Cell Carcinoma of the Esophagus[J]. Wei Xun Huan Xue Za Zhi, 2022, 32(4): 75-79, 84.] doi: 10.3969/j.issn.1005-1740.2022.04.015 Cai GK, Chen YS. The Research Progress of Treatment on Primary Small Cell Carcinoma of the Esophagus[J]. Wei Xun Huan Xue Za Zhi, 2022, 32(4): 75-79, 84. doi: 10.3969/j.issn.1005-1740.2022.04.015
[2] 郭小川, 邓汉宇. 可切除食管小细胞癌的临床特点及外科治疗预后分析[J]. 中国胸心血管外科临床杂志, 2019, 26(10): 992-997. [Guo XC, Deng HY. Clinical characteristics and prognosis of resectable esophagus small cell carcinoma after surgical resection[J]. Zhongguo Xiong Xin Xue Guan Wai Ke Lin Chuang Za Zhi, 2019, 26(10): 992-997.] doi: 10.7507/1007-4848.201902001 Guo XC, Deng HY. Clinical characteristics and prognosis of resectable esophagus small cell carcinoma after surgical resection[J]. Zhongguo Xiong Xin Xue Guan Wai Ke Lin Chuang Za Zhi, 2019, 26(10): 992-997. doi: 10.7507/1007-4848.201902001
[3] Xiao Q, Xiao H, Ouyang S, et al. Primary small cell carcinoma of the esophagus: Comparison between a Chinese cohort and Surveillance, Epidemiology, and End Results (SEER) data[J]. Cancer Med, 2019, 8(3): 1074-1085. doi: 10.1002/cam4.2001
[4] Ji A, Jin R, Zhang R, et al. Primary small cell carcinoma of the esophagus: progression in the last decade[J]. Ann Transl Med, 2020, 8(7): 502. doi: 10.21037/atm.2020.03.214
[5] Giannetta E, Guarnotta V, Rota F, et al. A rare rarity: Neuroendocrine tumor of the esophagus[J]. Cri Rev Oncol Hematol, 2019, 137: 92-107. doi: 10.1016/j.critrevonc.2019.02.012
[6] Xu L, Li Y, Liu X, et al. Treatment Strategies and Prognostic Factors of Limited-Stage Primary Small Cell Carcinoma of the Esophagus[J]. J Thorac Oncol, 2017, 12(12): 1834-1844. doi: 10.1016/j.jtho.2017.09.1966
[7] 张惠娟, 周胜理, 赵兰芳, 等. 食管原发性小细胞神经内分泌癌22例临床病理分析[J]. 中华肿瘤防治杂志, 2019, 26(16): 1200-1207. [Zhang HJ, Zhou SL, Zhao LF, et al. Clinical analysis of 22 cases with esophageal primary small cell neuroendocrine carcinoma[J]. Zhonghua Zhong Liu Fang Zhi Za Zhi, 2019, 26(16): 1200-1207.] Zhang HJ, Zhou SL, Zhao LF, et al. Clinical analysis of 22 cases with esophageal primary small cell neuroendocrine carcinoma[J]. Zhonghua Zhong Liu Fang Zhi Za Zhi, 2019, 26(16): 1200-1207.
[8] Zhu Y, Qiu B, Liu H, et al. Primary small cell carcinoma of the esophagus: review of 64 cases from a single institution[J]. Dis Esophagus, 2014, 27(2): 152-158. doi: 10.1111/dote.12069
[9] 周琳, 焦笑笑, 刘璐, 等. 中美249例原发性食管小细胞癌的临床病理特征对比、治疗方式及预后分析[J]. 郑州大学学报(医学版), 2020, 55(5): 598-602. [Zhou L, Jiao XX, Liu L, et al. Comparison of clinicopathological features, treatment modalities and prognosis of 249 primary small cell carcinomaof esophagus cases between China and the United States[J]. Zhengzhou Da Xue Xue Bao(Yi Xue Ban), 2020, 55(5): 598-602.] Zhou L, Jiao XX, Liu L, et al. Comparison of clinicopathological features, treatment modalities and prognosis of 249 primary small cell carcinomaof esophagus cases between China and the United States[J]. Zhengzhou Da Xue Xue Bao(Yi Xue Ban), 2020, 55(5): 598-602.
[10] Honma Y, Nagashima K, Hirano H, et al. Clinical outcomes of locally advanced esophageal neuroendocrine carcinoma treated with chemoradiotherapy[J]. Cancer Med, 2020, 9(2): 595-604. doi: 10.1002/cam4.2708
[11] 夏伊麦尔旦·伊不拉音, 宋朋, 高树庚. 原发性食管小细胞癌的治疗及预后分析[J]. 中华肿瘤杂志, 2020, 42(8): 670-675. [Yibulayin XYMED, Song P, Gao SG. Treatment and prognostic analysis of patients with primary esophageal small-cell carcinoma[J]. Zhonghua Zhong Liu Za Zhi, 2020, 42(8): 670-675.] doi: 10.3760/cma.j.cn112152-20191023-00680 Yibulayin XYMED, Song P, Gao SG. Treatment and prognostic analysis of patients with primary esophageal small-cell carcinoma[J]. Zhonghua Zhong Liu Za Zhi, 2020, 42(8): 670-675. doi: 10.3760/cma.j.cn112152-20191023-00680
[12] 张伟, 袁婕, 王捷忠. 51例食管小细胞癌非手术治疗患者的预后生存分析[J]. 中外医疗, 2023, 42(11): 34-38. [Zhang W, Yuan J, Wang JZ. Prognosis and survival analysis of 51 patients with esophageal small cell carcinoma treated without operation[J]. Zhong Wai Yi Liao, 2023, 42(11): 34-38.] Zhang W, Yuan J, Wang JZ. Prognosis and survival analysis of 51 patients with esophageal small cell carcinoma treated without operation[J]. Zhong Wai Yi Liao, 2023, 42(11): 34-38.
[13] 王玉栋, 焦文鹏, 王军, 等. 老年局限期食管小细胞癌临床病理特征及预后分析[J]. 中华肿瘤防治杂志, 2016, 23(18): 1254-1259. [Wang YD, Jiao WP, Wang J, et al. Clinicopathological characteristics and prognosis analysis in the elderly patients with primary esophageal small cell carcinoma in limited stage[J]. Zhonghua Zhong Liu Fang Zhi Za Zhi, 2016, 23(18): 1254-1259.] Wang YD, Jiao WP, Wang J, et al. Clinicopathological characteristics and prognosis analysis in the elderly patients with primary esophageal small cell carcinoma in limited stage[J]. Zhonghua Zhong Liu Fang Zhi Za Zhi, 2016, 23(18): 1254-1259.
[14] 王军, 焦文鹏, 曹峰, 等. 121例局限期食管小细胞癌治疗模式探讨[J]. 中华放射肿瘤学杂志, 2016, 25(7): 699-702. [Wang J, Jiao WP, Cao F, et al. Treatment modalities for limited-stage small-cell esophageal cancer: an analysis of 121 patients[J]. Zhonghua Fang She Zhong Liu Xue Za Zhi, 2016, 25(7): 699-702.] doi: 10.3760/cma.j.issn.1004-4221.2016.07.008 Wang J, Jiao WP, Cao F, et al. Treatment modalities for limited-stage small-cell esophageal cancer: an analysis of 121 patients[J]. Zhonghua Fang She Zhong Liu Xue Za Zhi, 2016, 25(7): 699-702. doi: 10.3760/cma.j.issn.1004-4221.2016.07.008
[15] Jeene PM, Geijsen ED, Muijs CT, et al. Small Cell Carcinoma of the Esophagus: A Nationwide Analysis of Treatment and Outcome at Patient Level in Locoregional Disease[J]. Am J Clin Oncol, 2019, 42(6): 534-538. doi: 10.1097/COC.0000000000000546
[16] 侯宏霖, 聂彩云, 王茂勋, 等. 原发性食管小细胞癌82例临床特点分析[J]. 中国医学前沿杂志(电子版), 2020, 12(3): 72-77. [Hou HL, Nie CY, Wang MX, et al. Analysis of clinical features of 82 cases of primary esophageal small cell carcinoma[J]. Zhongguo Yi Xue Qian Yan Za Zhi(Dian Zi Ban), 2020, 12(3): 72-77.] Hou HL, Nie CY, Wang MX, et al. Analysis of clinical features of 82 cases of primary esophageal small cell carcinoma[J]. Zhongguo Yi Xue Qian Yan Za Zhi(Dian Zi Ban), 2020, 12(3): 72-77.
[17] 孙亚星, 李建生, 吕笑娟, 等. 食管原发性小细胞神经内分泌癌78例临床特点及预后分析[J]. 胃肠病学和肝病学杂志, 2018, 27(5): 539-542. [Sun YX, Li JS, Lu XJ, et al. Clinical features and prognosis analysis of 78 patients with esophageal primary small cell neuroendocrine carcinoma[J]. Wei Chang Bing Xue He Gan Bing Xue Za Zhi, 2018, 27(5): 539-542.] doi: 10.3969/j.issn.1006-5709.2018.05.015 Sun YX, Li JS, Lu XJ, et al. Clinical features and prognosis analysis of 78 patients with esophageal primary small cell neuroendocrine carcinoma[J]. Wei Chang Bing Xue He Gan Bing Xue Za Zhi, 2018, 27(5): 539-542. doi: 10.3969/j.issn.1006-5709.2018.05.015
[18] 唐文超, 李远伟, 陈佳, 等. 术前预后营养指数及中性粒-淋巴细胞比值在非肌层浸润性膀胱癌患者复发中的预测价值[J]. 现代肿瘤医学, 2022, 30(12): 2218-2223. [Tang WC, Li YW, Chen J, et al. The predictive value of preoperative PNI and NLR for recurrence of non-muscular invasive bladder cancer[J]. Xian Dai Zhong Liu Yi Xue, 2022, 30(12): 2218-2223.] doi: 10.3969/j.issn.1672-4992.2022.12.022 Tang WC, Li YW, Chen J, et al. The predictive value of preoperative PNI and NLR for recurrence of non-muscular invasive bladder cancer[J]. Xian Dai Zhong Liu Yi Xue, 2022, 30(12): 2218-2223. doi: 10.3969/j.issn.1672-4992.2022.12.022
[19] 何杰, 王莉, 徐卫. 预后营养指数在恶性淋巴瘤患者中的预后价值[J]. 中国实验血液学杂志, 2022, 30(2): 641-644. [He J, Wang L, Xu W. The Prognostic Value of Prognostic Nutritional Index in Patients with Lymphoma—Review[J]. Zhongguo Shi Yan Xue Ye Xue Za Zhi, 2022, 30(2): 641-644.] He J, Wang L, Xu W. The Prognostic Value of Prognostic Nutritional Index in Patients with Lymphoma—Review[J]. Zhongguo Shi Yan Xue Ye Xue Za Zhi, 2022, 30(2): 641-644.
[20] 毛文杰, 尹泚, 李斌, 等. 术前炎症免疫及营养指标对胸腺瘤患者预后的预测价值[J]. 解放军医学杂志, 2021, 46(11): 1104-1111. [Mao WJ, Ying C, Li B, et al. Predictive value of preoperative inflammatory immune and nutritional indicators for the prognosis of patients with thymoma[J]. Jie Fang Jun Yi Xue Za Zhi, 2021, 46(11): 1104-1111.] doi: 10.11855/j.issn.0577-7402.2021.11.07 Mao WJ, Ying C, Li B, et al. Predictive value of preoperative inflammatory immune and nutritional indicators for the prognosis of patients with thymoma[J]. Jie Fang Jun Yi Xue Za Zhi, 2021, 46(11): 1104-1111. doi: 10.11855/j.issn.0577-7402.2021.11.07
[21] 俞璐莎, 高丽渊, 黄应亮. 基于系统免疫炎症指数和预后营养指数预测喉癌患者预后[J]. 疾病监测, 2024, 39(1): 108-114. [Yu LS, Gao LY, Huang YL. Study of relationship between systemic immune inflammatory index, prognostic nutritional index and prognosis of patients with laryngeal cancer[J]. Ji Bing Jian Ce, 2024, 39(1): 108-114.] doi: 10.3784/jbjc.202303180111 Yu LS, Gao LY, Huang YL. Study of relationship between systemic immune inflammatory index, prognostic nutritional index and prognosis of patients with laryngeal cancer[J]. Ji Bing Jian Ce, 2024, 39(1): 108-114. doi: 10.3784/jbjc.202303180111
[22] Kubota K, Ito R, Narita N, et al. Utility of prognostic nutritional index and systemic immune-inflammation index in oral cancer treatment[J]. BMC Cancer, 2022, 22(1): 368. doi: 10.1186/s12885-022-09439-x
[23] Meng L, Yang Y, Hu X, et al. Prognostic value of the pretreatment systemic immune-inflammation index in patients with prostate cancer: a systematic review and meta-analysis[J]. J Transl Med, 2023, 21(1): 79. doi: 10.1186/s12967-023-03924-y
[24] 马海燕, 冯海红, 郭飞, 等. 结直肠癌患者血清总蛋白、白蛋白、血红蛋白与Th1/Th2免疫平衡及调节性T细胞、辅助性T细胞17相关性分析[J]. 中国医药导报, 2023, 20(11): 106-110, 122. [Ma HY, Feng HH, Guo F, et al. Correlation analysis of serum total protein, albumin and hemoglobin with T cell immune balance, regulatory cell and T helper cell 17 in patients with colorectal cancer[J]. Zhongguo Yi Yao Dao Bao, 2023, 20(11): 106-110, 122.] Ma HY, Feng HH, Guo F, et al. Correlation analysis of serum total protein, albumin and hemoglobin with T cell immune balance, regulatory cell and T helper cell 17 in patients with colorectal cancer[J]. Zhongguo Yi Yao Dao Bao, 2023, 20(11): 106-110, 122.
[25] Xiong T, He P, Zhou M, et al. Glutamate blunts cell-killing effects of neutrophils in tumor microenvironment[J]. Cancer Science, 2022, 113(6): 1955-1967. doi: 10.1111/cas.15355
[26] 罗苏亚, 蒋欣. 血小板计数及纤维蛋白原水平与卵巢上皮性癌临床病理因素的 相关性及其对预后的影响[J]. 临床与病理杂志, 2017, 37(9): 1866-1873. [Luo SY, Jiang X. Prevalence and prognostic value of platelets and fibrinogen in preoperative patients with epithelial ovarian cancer[J]. Lin Chuang Yu Bing Li Za Zhi, 2017, 37(9): 1866-1873.] Luo SY, Jiang X. Prevalence and prognostic value of platelets and fibrinogen in preoperative patients with epithelial ovarian cancer[J]. Lin Chuang Yu Bing Li Za Zhi, 2017, 37(9): 1866-1873.
[27] 苏比努尔·依孜哈尔, 达尼亚尔·努尔德别克, 玛依努尔·艾力. 炎症及营养指标与食管癌患者预后相关性分析[J]. 中国临床医生杂志, 2024, 52(1): 39-43. [Yizihaer SBNE, Nuerdebieke DNYE, Aili MYNE. Analysis of the correlation between inflammation and nutrition indexes and prognosis of patients with esophageal cancer[J]. Zhongguo Lin Chuang Yi Sheng Za Zhi, 2024, 52(1): 39-43.] Yizihaer SBNE, Nuerdebieke DNYE, Aili MYNE. Analysis of the correlation between inflammation and nutrition indexes and prognosis of patients with esophageal cancer[J]. Zhongguo Lin Chuang Yi Sheng Za Zhi, 2024, 52(1): 39-43.
[28] Wang N, Li X, Luo H, et al. Prognostic value of pretreatment inflammatory biomarkers in primary small cell carcinoma of the esophagus[J]. Thorac Cancer, 2019, 10(10): 1913-1918. doi: 10.1111/1759-7714.13164
[29] 胡小秀, 何义富, 罗会芹, 等. 外周血NLR、PLR与食管小细胞癌临床预后的关系[J]. 临床肿瘤学杂志, 2019, 24(6): 543-547. [Hu XX, He YF, Luo HQ, et al. The relationship between peripheral blood NLR, PLR and clinical prognosis of small cell esophageal carcer[J]. Lin Chuang Zhong Liu Xue Za Zhi, 2019, 24(6): 543-547.] Hu XX, He YF, Luo HQ, et al. The relationship between peripheral blood NLR, PLR and clinical prognosis of small cell esophageal carcer[J]. Lin Chuang Zhong Liu Xue Za Zhi, 2019, 24(6): 543-547.
[30] 郁文恺, 陈健琳, 王建芳, 等. 实验室检查指标与晚期癌症患者生存情况的关系研究[J]. 中国全科医学, 2021, 24(21): 2691-2695. [Yu WK, Chen JL, Wang JF, et al. Laboratory Test Indices and Survival Status in Patients with Advanced Cancer[J]. Zhongguo Quan Ke Yi Xue, 2021, 24(21): 2691-2695.] Yu WK, Chen JL, Wang JF, et al. Laboratory Test Indices and Survival Status in Patients with Advanced Cancer[J]. Zhongguo Quan Ke Yi Xue, 2021, 24(21): 2691-2695.
[31] 施毓婷, 王凯, 赵江南, 等. C反应蛋白与白蛋白相关免疫营养参数在肺癌中的研究进展[J]. 国际呼吸杂志, 2022, 42(17): 1350-1355. [Shi YT, Wang K, Zhao JN, et al. Research progress of C-reactive protein and albumin related immune-nutrition parameters in lung cancer[J]. Guo Ji Hu Xi Za Zhi, 2022, 42(17): 1350-1355.] doi: 10.3760/cma.j.cn131368-20220623-00531 Shi YT, Wang K, Zhao JN, et al. Research progress of C-reactive protein and albumin related immune-nutrition parameters in lung cancer[J]. Guo Ji Hu Xi Za Zhi, 2022, 42(17): 1350-1355. doi: 10.3760/cma.j.cn131368-20220623-00531
[32] Yamashita S, Abe H, Yamashita H, et al. PD-L1 and HLA-class I expression status and their therapeutic implication in oesophageal small-cell carcinoma[J]. Histopathology, 2023, 83(2): 264-275. doi: 10.1111/his.14924



 下载:
下载: