Relationship Between Peripheral Blood T Lymphocyte Subsets and Prognosis of Patients with Advanced Non-small Cell Lung Cancer Treated with Camrelizumab
-
摘要:目的
探索外周血T淋巴细胞亚群与接受卡瑞利珠单抗治疗的晚期非小细胞肺癌(NSCLC)患者预后的关系。
方法回顾性收集接受卡瑞利珠单抗治疗的88例晚期NSCLC患者资料。分别收集患者治疗前和治疗后2个月的外周血淋巴细胞亚群相对计数,使用Kaplan-Meier曲线和Cox回归分析研究外周血T淋巴细胞亚群与PFS和OS之间的关系。
结果有反应组患者治疗前外周血CD4+/CD8+比值高于无反应组(P=0.038),而CD8+T淋巴细胞百分比低于无反应组(P=0.036);生存分析显示治疗前CD4+/CD8+比值越高,患者PFS和OS越长(P=0.001,P=0.023)。多变量Cox分析显示,治疗前CD4+/CD8+比值是预测PFS和OS的因素。此外,治疗后CD4+/CD8+比值、CD4+T淋巴细胞百分比越高,患者PFS越长(P=0.005,0.015);CD8+T淋巴细胞百分比越低,患者PFS和OS越长(P=0.001,P=0.016)。
结论外周血CD4+/CD8+比值能够预测接受卡瑞利珠单抗治疗的NSCLC患者的生存情况。
Abstract:ObjectiveTo explore the relationship between peripheral blood T lymphocyte subsets and prognosis of patients with advanced non-small cell lung cancer (NSCLC) who received treatment with camrelizumab.
MethodsWe retrospectively collected data from 88 patients with advanced NSCLC who underwent camrelizumab treatment. Peripheral blood lymphocyte subsets were collected from patients before and two months after treatment. Kaplan-Meier curves and Cox regression analysis were employed to investigate the relationship between peripheral blood T lymphocyte subsets and PFS and OS.
ResultsCompared with non-responder group, the baseline peripheral blood CD4+/CD8+ ratio was higher (P=0.038), while the CD8+T lymphocyte percentage was lower (P=0.036) in the responder group. Kaplan-Meier curves showed that a high baseline CD4+/CD8+ ratio was associated with long PFS and OS (P=0.001, P=0.023). Multivariate Cox analysis revealed that the baseline CD4+/CD8+ ratio was a significant predictor for PFS and OS. Additionally, a high post-treatment CD4+/CD8+ ratio and high CD4+T lymphocyte percentage were associated with long PFS (P=0.005, P=0.015), whereas a low post-treatment CD8+T lymphocyte percentage was associated with long PFS and OS (P=0.001, P=0.016).
ConclusionThe peripheral blood CD4+/CD8+ ratio can serve as a predictive factor for survival of patients with NSCLC treated with camrelizumab.
-
Key words:
- Non-small cell lung cancer /
- CD4+/CD8+ ratio /
- CD4+T cell /
- CD8+T cell /
- Anti-PD-1 immunotherapy /
- Camrelizumab
-
0 引言
肺癌是全球范围内发病率第二、死亡率第一的恶性肿瘤[1]。其中,非小细胞肺癌(non-small cell lung cancer, NSCLC)约占全部肺癌病例的85%。近年来,针对程序性死亡受体1(programmed death 1, PD-1)及其配体(programmed death-ligand 1, PD-L1)的免疫检查点抑制剂(immune checkpoint inhibitors, ICIs)在NSCLC的治疗中取得了显著的疗效,如帕博利珠单抗、阿替利珠单抗和卡瑞利珠单抗等[2-5]。尽管多种ICIs已被批准作为NSCLC的一线治疗,仍有部分患者治疗效果不理想。因此,近来许多研究致力于探索能够预测治疗疗效的生物标志物[6]。目前发现PD-L1表达、肿瘤突变负荷、肿瘤浸润淋巴细胞与免疫治疗的临床效果相关,然而,这些基于组织的生物标志物在实际应用中仍存在一些困难,例如组织标本获取难度大、检测费用较高、无法实现动态监测等[7-9]。
外周血生物标志物具有相对容易获取、创伤小以及可重复取样的特点,因此可能是一种潜在的有效评估PD-L1/PD-1抑制剂疗效的手段[10]。已有研究发现,多种外周血标志物与ICIs治疗NSCLC患者的疗效相关,如嗜中性粒细胞与淋巴细胞比值、中性粒细胞绝对计数、C-反应蛋白和乳酸脱氢酶等全身炎性反应指标[11-15]。然而,关于外周血T淋巴细胞与NSCLC患者生存期的研究较少,且尚未得到统一的结论。既往一项使用PD-1/PD-L1抑制剂治疗NSCLC的研究发现,外周血CD4+T细胞与CD8+T细胞的百分比越高,患者生存期越长[16]。然而,另一项研究发现,治疗前外周血CD8+T细胞水平越低,临床效果越好[17]。因此,外周血T淋巴细胞在评估抗PD-1/PD-L1疗效中的应用价值仍值得进一步探讨。本研究旨在探讨ICIs治疗前后外周血T淋巴细胞亚群对于患者生存期的潜在预测价值。
1 资料与方法
1.1 研究对象
本研究纳入2020年1月至2021年12月期间来自连云港市第一人民医院共计88例NSCLC患者。纳入标准:(1)年龄为18~80周岁;(2)组织学或细胞学确诊为NSCLC;(3)肿瘤TNM分期为Ⅲ~Ⅳ期;(4)患者接受卡瑞利珠单抗治疗,包括卡瑞利珠单抗单药治疗或卡瑞利珠单抗联合化疗;(5)至少存在一个可测量病灶。排除标准:(1)人类免疫缺陷病毒阳性的患者;(2)接受皮质类固醇治疗的患者;(3)接受免疫抑制治疗的患者。随访截至2023年7月31日,中位随访时间11.3个月。本研究获得连云港市第一人民医院伦理委员会批准。
收集患者的临床病理资料,包括年龄、性别、肿瘤分期、组织病理类型、驱动基因突变状态等;分别收集患者治疗前和3周期治疗后外周血T淋巴细胞百分比,包括CD4+T淋巴细胞百分比、CD8+T淋巴细胞百分比和CD4+/CD8+比值。
1.2 疗效评价
根据实体瘤疗效评价标准1.1对疗效进行评价。以基线总直径作为参考,完全缓解(complete response, CR)指所有目标病灶完全消失;部分缓解(partial response, PR)指目标病灶总直径至少减少30%;进展(progressive disease, PD)是指目标病灶总直径至少增加20%,并且总直径绝对值至少增加5 mm;疾病稳定(stable disease, SD)指病灶缩小未达到PR的程度或增大未达到PD程度。无进展生存期(progression-free survival, PFS)定义为从免疫治疗开始到疾病进展或死亡的时间。总生存期(overall survival, OS)定义为从免疫治疗开始到任何原因死亡的时间。
1.3 统计学方法
统计分析利用SPSS Statistics 26软件完成。分别使用Mann-Whitney U检验对连续变量进行比较。以中位值为临界值分为高、低水平组。采用单变量Cox回归模型估计临床参数和外周血指标的生存风险比(hazard ratio, HR)和95%置信区间(confidence interval, CI)。使用Kaplan-Meier方法进行生存分析,并通过Log rank检验比较P值。多变量Cox分析仅包括在单变量分析中具有统计学意义的参数。采用多变量Cox回归模型估计生存的HR和95%CI。P<0.05为差异有统计学意义。
2 结果
2.1 临床资料
本研究共纳入88例接受卡瑞利珠单抗治疗的NSCLC患者,临床资料见表1。患者中位年龄63岁(45~82岁),其中男性67例,女性21例,肺鳞癌24例,肺腺癌64例。其中72例患者进行了基因检测,14例存在表皮生长因子受体(epidermal growth factor receptor, EGFR)或无间变淋巴瘤激酶(anaplastic lymphoma kinase, ALK) 基因突变。33例患者进行了PD-L1检测,24例表达阳性,9例表达阴性。根据RECIST1.1标准进行疗效评估,CR患者0例,PR患者48例,SD患者22例,PD患者18例,客观缓解率(objective response rate, ORR)为54.5%,疾病控制率(disease control rate, DCR)为79.5%。
表 1 88例患者的临床和病理特征资料Table 1 Clinical and pathological characteristics of the participantsClinical characteristics N=88 Percentage(%) Age, median (range) 63 45-82 Gender Male 67 76.10 Female 21 23.90 Histology Squamous 24 27.30 Adenocarcinoma 64 72.70 Driver gene EGFR or ALK 14 15.90 Wild type 58 65.90 Unknown 16 18.20 PD-L1 Negative 9 10.20 Positive 24 27.30 Unknown 55 62.50 Treatment line 1st line 43 48.90 ≥2nd line 45 51.10 Tumor stage Ⅲ 16 18.20 Ⅳ 72 81.80 2.2 外周血标志物与治疗反应的关系
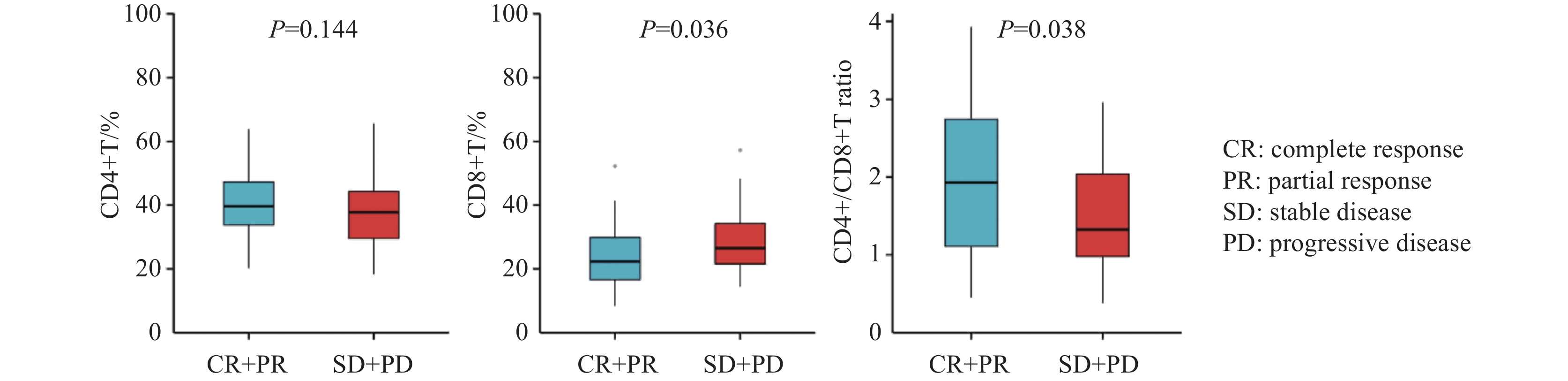
根据疗效将患者分为有反应组(CR+PR)和无反应组(SD+PD)。结果显示,有反应组治疗前CD4+T淋巴细胞百分比较无反应组升高,但差异无统计学意义(中位值:39.61% vs. 37.74%,P=0.144),见图1A。与无反应组相比,有反应组治疗前CD8+T淋巴细胞百分比显著下降(中位值:22.3% vs. 26.49%,P=0.036),见图1B,CD4+/CD8+比值显著升高(中位值:1.93 vs. 1.33,P=0.038),见图1C。
2.3 治疗前外周血标志物与生存的关系
治疗前CD3+T细胞百分比中位值为68.83%,CD4+T细胞百分比中位值为39.56%,CD8+T细胞百分比中位值为24.43%,CD4+/CD8+比值中位值为1.56。使用单变量Cox分析与患者PFS、OS相关的因素。单变量Cox分析中包括6个临床指标和4个外周血指标,在PFS的单变量Cox分析中,年龄、性别、肿瘤分期、病理类型、驱动基因状态、CD3+T细胞百分比均与PFS无关;治疗线数(P=0.03)、CD4+T细胞百分比(P=0.004)、CD4+/CD8+比值(P=0.001)和CD8+T细胞百分比(P=0.002)与患者的PFS显著相关,见表2。在OS的单变量Cox分析中,CD8+T细胞百分比(P=0.026,HR=1.754,95%CI:1.070~2.875)、CD4+/CD8+比值(P=0.025,HR=0.564,95%CI:0.342~0.930)与患者OS相关,见表3。
表 2 患者无进展生存期与临床病理特征关系的Cox分析Table 2 Association between progression-free survival and clinicopathologic features using Cox regressionClinical
characteristicsUnivariate Cox Multivariate Cox P HR 95%CI P HR 95%CI Age (years) >65 vs. ≤65 0.924 1.022 0.652–1.602 − − − Gender Male vs.
Female0.225 0.728 0.435–1.216 − − − Tumor stage Ⅳ vs. Ⅲ 0.150 1.576 0.848–2.929 − − − Histological type Adenocarcinoma
vs. Squamous0.066 1.634 0.969–2.755 − − − Treatment line ≥2nd line
vs. 1st line0.030 1.662 1.051–2.629 0.255 − − Driver gene
(EGFR/ALK)Mutation
vs. Wild0.754 0.907 0.492–1.672 − − − Unknown
vs. Wild0.078 0.576 0.312–1.064 − − − CD3+T (%) High vs. Low 0.445 1.194 0.758–1.882 − − − CD4+T (%) High vs. Low 0.004 0.501 0.314–0.801 0.106 − − CD8+T (%) High vs. Low 0.002 2.110 1.325–3.36 0.502 − − CD4+/CD8+ High vs. Low 0.001 0.451 0.283–0.72 0.001 0.451 0.283–0.72 Notes: HR: hazard ratio; CI: confidence interval; −: no data. 表 3 患者总生存期与临床病理特征关系的Cox分析Table 3 Association between overall survival and clinicopathologic features using Cox regressionClinical
characteristicsUnivariate Cox Multivariate Cox P HR 95%CI P HR 95%CI Age (years) >65 vs. ≤65 0.838 1.052 0.646–1.713 − − − Gender Male vs.
Female0.625 0.869 0.494–1.529 − − − Tumor stage Ⅳ vs. Ⅲ 0.198 1.560 0.793–3.070 − − − Histological
typeAdenocarcinoma
vs. Squamous0.272 1.373 0.780–2.418 − − − Treatment
line≥2nd line
vs. 1st line0.829 1.055 0.648–1.717 − − − Driver gene
(EGFR/ALK)Mutation
vs. Wild0.412 0.740 0.361–1.518 − − − Unknown
vs. Wild0.790 0.917 0.484–1.737 − − − CD3+T (%) High vs. Low 0.271 1.316 0.807–2.143 − − − CD4+T (%) High vs. Low 0.115 0.672 0.410–1.101 − − − CD8+T (%) High vs. Low 0.026 1.754 1.070–2.875 0.51 − − CD4+/CD8+ High vs. Low 0.025 0.564 0.342–0.930 0.025 0.564 0.342–0.93 Notes: HR: hazard ratio; CI: confidence interval; −: no data. 在多变量Cox回归分析中,CD4+/CD8+比值是PFS的独立预测因素(P=0.001,HR=0.451,95%CI:0.283-0.720),高水平CD4+/CD8+比值是较长OS的预测因素(P=0.025,HR=0.564,95%CI:0.342~0.93)。
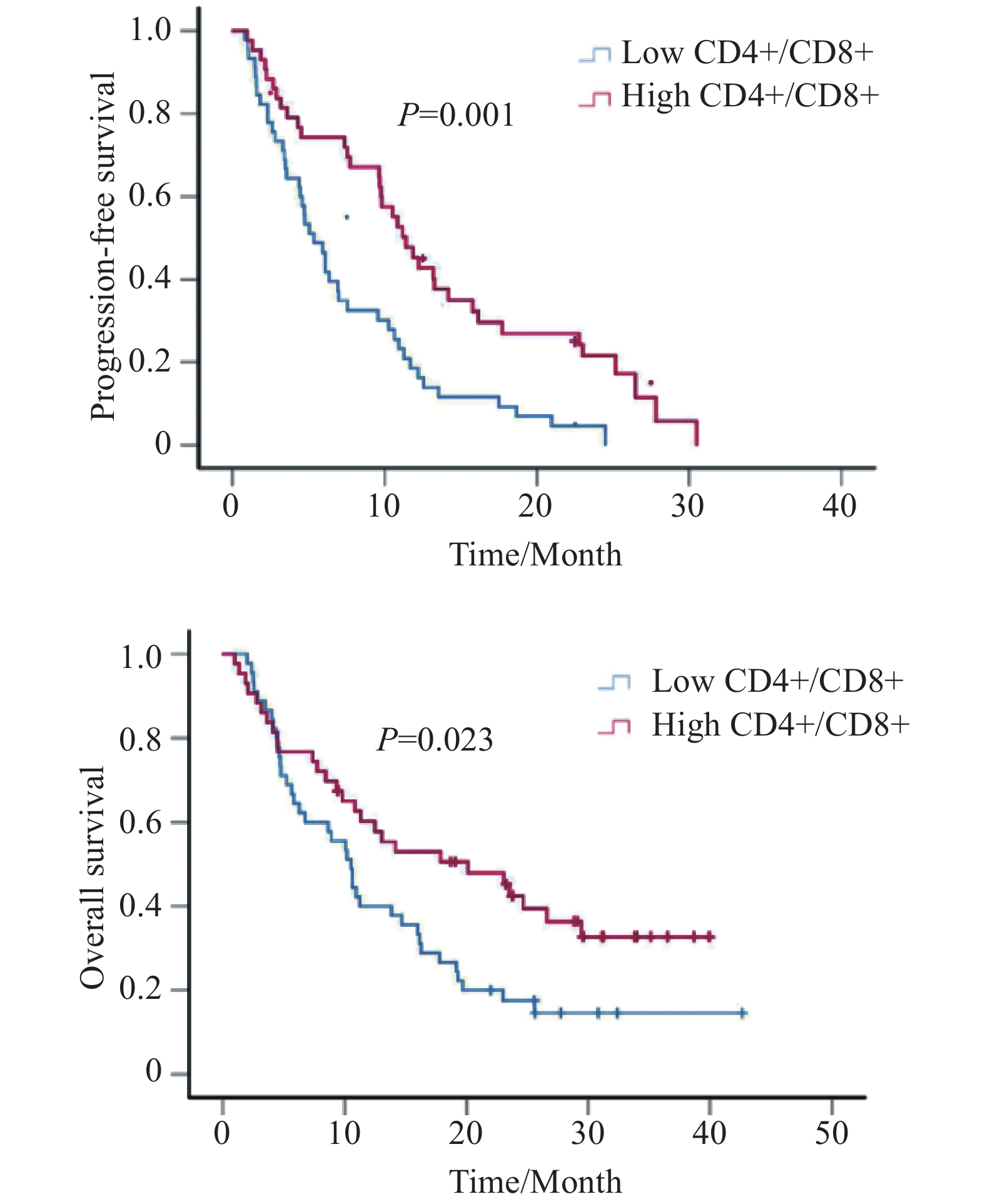
Kaplan-Meier生存分析显示,CD4+/CD8+水平越高,患者PFS(中位PFS:5.3 vs. 11.4月,P=0.001)和OS越长(中位OS:10.5 vs. 20.1月,P=0.023),差异有统计学意义,见图2。
2.4 治疗2月后外周血的标志物与生存的关系
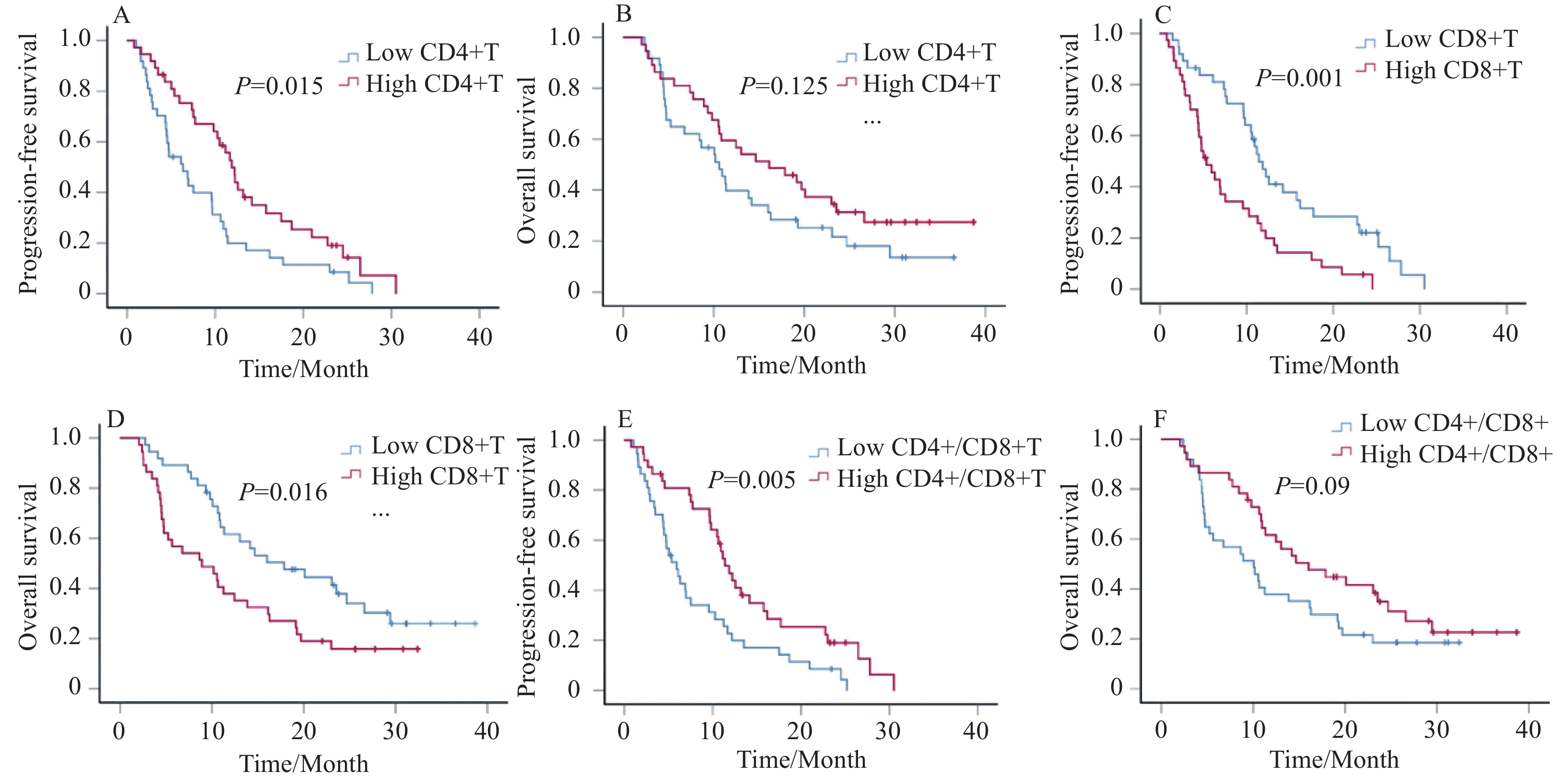
收集患者在治疗2月后的外周血T淋巴细胞亚群相对计数,根据中位值将其分为高水平和低水平组。治疗后的CD3+T细胞百分比、CD4+T细胞百分比、CD8+T细胞百分比、CD4+/CD8+比值的中位值分别为71.66%、39.68%、25.75%和1.52。Kaplan-Meier生存曲线显示治疗后CD4+T细胞百分比越高,PFS越长(中位PFS:6.3 vs. 11.9月,P=0.015),见图3A。相较于CD8+T高水平组,低水平CD8+T组患者的PFS和OS显著延长(中位PFS:5.4 vs. 11.4月,P=0.001;中位OS:8.9 vs. 17.9月,P=0.016),见图3C、D。治疗后CD4+/CD8+比值越高,患者PFS越长(中位PFS:5.9 vs. 11.4月,P=0.005),OS越长(中位OS:10.1 vs. 16月,P=0.09),见图3E、F。
![]() 图 3 治疗2月后CD4+T、CD8+T和CD4+/CD8+比值与患者PFS、OS的Kaplan–Meier生存曲线Figure 3 Kaplan–Meier survival curves for PFS and OS according to CD4+T, CD8+T, and CD4+/CD8+ ratio after two months treatmentA, B: PFS and OS in the high CD4+T group and in the low CD4+T group after treatment; C, D: PFS and OS in the high CD8+T group and in the low CD8+T group after treatment; E, F: PFS and OS in the high CD4+/CD8+ ratio group and in the low CD4+/CD8+ ratio group after treatment.
图 3 治疗2月后CD4+T、CD8+T和CD4+/CD8+比值与患者PFS、OS的Kaplan–Meier生存曲线Figure 3 Kaplan–Meier survival curves for PFS and OS according to CD4+T, CD8+T, and CD4+/CD8+ ratio after two months treatmentA, B: PFS and OS in the high CD4+T group and in the low CD4+T group after treatment; C, D: PFS and OS in the high CD8+T group and in the low CD8+T group after treatment; E, F: PFS and OS in the high CD4+/CD8+ ratio group and in the low CD4+/CD8+ ratio group after treatment.3 讨论
本研究回顾性探讨了接受卡瑞利珠单抗治疗的NSCLC患者外周血T淋巴细胞亚群的预后预测价值。研究发现CD4+/CD8+比值是接受卡瑞利珠单抗治疗的NSCLC患者PFS和OS的预测因素。此外,患者接受PD-1抑制剂治疗前外周血CD4+/CD8+比值可能与肿瘤反应有关。
大量的淋巴细胞浸润是抗肿瘤免疫的基础,因此,监测淋巴细胞亚群对抗肿瘤治疗的疗效判断具有重要意义。淋巴细胞根据其表面标志物可分为T淋巴细胞、B淋巴细胞和自然杀伤细胞。其中,T淋巴细胞是淋巴细胞亚群的重要组成部分。CD4+T淋巴细胞能分化为辅助性T细胞亚群(Th1、Th2、Th17)和调节性T细胞[18],大部分CD4+T细胞发挥抗肿瘤活性。CD8+T细胞包括Tc细胞和Ts细胞,Tc细胞发挥细胞毒性作用,而Ts细胞抑制CD4+T细胞的活化和增殖[19]。CD4+/CD8+比值能够反映机体免疫状态,当比值下降时意味着细胞免疫功能下降,对肿瘤杀伤作用减弱,使机体处于免疫抑制状态[20]。CD4+T细胞、CD8+T细胞等淋巴细胞在抗肿瘤免疫中起着重要作用。
既往有研究探索了PD-1抑制剂治疗后外周血T淋巴细胞的变化,在PD-1抑制剂治疗后,患者外周血中CD4+T淋巴细胞的数量增加,细胞免疫功能也得到改善[21]。也有研究探讨外周血淋巴细胞与ICIs治疗晚期NSCLC疗效之间的关系,主要研究的药物包括纳武利尤单抗和帕博利珠单抗[16, 22],但鲜有关于卡瑞利珠单抗的报道。因此,本研究聚焦于卡瑞利珠单抗治疗疗效与T淋巴细胞亚群之间的关系。结果发现,在接受卡瑞利珠单抗治疗之前,外周血中的CD4+/CD8+比值越低,患者的PFS越短,与我们既往研究发现治疗前CD4+/CD8+比值低与NSCLC患者不良预后相关的结果类似[23]。此外,Li等的研究结果显示,治疗前CD4+/CD8+比值越低,ICIs治疗的PFS更短[24],但是未对患者OS进行分析。因此,在本研究中,我们进一步统计了患者的OS,结果显示治疗前的CD4+/CD8+比值同样能够预测卡瑞利珠单抗治疗患者的OS。本研究还发现,在接受了2个月卡瑞利珠单抗治疗后,CD4+T淋巴细胞依然保持较高水平与患者较长的生存期有关。与此类似的是,Liu等也发现在接受ICIs治疗的胃肠癌患者中,短期治疗后CD4+T细胞可以作为预后标志物[25]。除此之外,本研究显示患者治疗前CD4+/CD8+比值和CD8+T淋巴细胞百分比与患者肿瘤反应相关。CD8+T细胞一方面对抗原提呈细胞有细胞毒性作用,另一方面还能通过产生抑制性细胞因子,抑制CD4+T细胞的抗肿瘤作用[26]。因此,高水平的CD8+T细胞可能与较差的肿瘤反应相关。
综上所述,外周血CD4+/CD8+比值能够预测接受卡瑞利珠单抗治疗的NSCLC患者的生存情况,外周血T淋巴细胞百分比有望成为预测抗PD-1/PD-L1治疗疗效的生物标志物。本研究仍存在一些局限性,缺乏外周血T淋巴细胞绝对计数的数据,百分比和绝对计数的联合可能对疗效具有更准确的预测价值。另外,本研究是一项回顾性研究,样本量较小,未来需要开展大样本的前瞻性研究来进一步验证研究结果。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:董长鸿:研究设计与实施、数据分析、论文撰写与修改封 岩:研究设计、临床数据筛选江艳婷:数据收集、统计学分析高 洁:数据收集、论文修改蒋晓东:研究设计、数据审核、论文修改 -
表 1 88例患者的临床和病理特征资料
Table 1 Clinical and pathological characteristics of the participants
Clinical characteristics N=88 Percentage(%) Age, median (range) 63 45-82 Gender Male 67 76.10 Female 21 23.90 Histology Squamous 24 27.30 Adenocarcinoma 64 72.70 Driver gene EGFR or ALK 14 15.90 Wild type 58 65.90 Unknown 16 18.20 PD-L1 Negative 9 10.20 Positive 24 27.30 Unknown 55 62.50 Treatment line 1st line 43 48.90 ≥2nd line 45 51.10 Tumor stage Ⅲ 16 18.20 Ⅳ 72 81.80 表 2 患者无进展生存期与临床病理特征关系的Cox分析
Table 2 Association between progression-free survival and clinicopathologic features using Cox regression
Clinical
characteristicsUnivariate Cox Multivariate Cox P HR 95%CI P HR 95%CI Age (years) >65 vs. ≤65 0.924 1.022 0.652–1.602 − − − Gender Male vs.
Female0.225 0.728 0.435–1.216 − − − Tumor stage Ⅳ vs. Ⅲ 0.150 1.576 0.848–2.929 − − − Histological type Adenocarcinoma
vs. Squamous0.066 1.634 0.969–2.755 − − − Treatment line ≥2nd line
vs. 1st line0.030 1.662 1.051–2.629 0.255 − − Driver gene
(EGFR/ALK)Mutation
vs. Wild0.754 0.907 0.492–1.672 − − − Unknown
vs. Wild0.078 0.576 0.312–1.064 − − − CD3+T (%) High vs. Low 0.445 1.194 0.758–1.882 − − − CD4+T (%) High vs. Low 0.004 0.501 0.314–0.801 0.106 − − CD8+T (%) High vs. Low 0.002 2.110 1.325–3.36 0.502 − − CD4+/CD8+ High vs. Low 0.001 0.451 0.283–0.72 0.001 0.451 0.283–0.72 Notes: HR: hazard ratio; CI: confidence interval; −: no data. 表 3 患者总生存期与临床病理特征关系的Cox分析
Table 3 Association between overall survival and clinicopathologic features using Cox regression
Clinical
characteristicsUnivariate Cox Multivariate Cox P HR 95%CI P HR 95%CI Age (years) >65 vs. ≤65 0.838 1.052 0.646–1.713 − − − Gender Male vs.
Female0.625 0.869 0.494–1.529 − − − Tumor stage Ⅳ vs. Ⅲ 0.198 1.560 0.793–3.070 − − − Histological
typeAdenocarcinoma
vs. Squamous0.272 1.373 0.780–2.418 − − − Treatment
line≥2nd line
vs. 1st line0.829 1.055 0.648–1.717 − − − Driver gene
(EGFR/ALK)Mutation
vs. Wild0.412 0.740 0.361–1.518 − − − Unknown
vs. Wild0.790 0.917 0.484–1.737 − − − CD3+T (%) High vs. Low 0.271 1.316 0.807–2.143 − − − CD4+T (%) High vs. Low 0.115 0.672 0.410–1.101 − − − CD8+T (%) High vs. Low 0.026 1.754 1.070–2.875 0.51 − − CD4+/CD8+ High vs. Low 0.025 0.564 0.342–0.930 0.025 0.564 0.342–0.93 Notes: HR: hazard ratio; CI: confidence interval; −: no data. -
[1] Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. doi: 10.3322/caac.21660
[2] Kim R, Keam B, Hahn S, et al. First-line Pembrolizumab Versus Pembrolizumab Plus Chemotherapy Versus Chemotherapy Alone in Non-small-cell Lung Cancer: A Systematic Review and Network Meta-analysis[J]. Clin Lung Cancer, 2019, 20(5): 331-338, e334. doi: 10.1016/j.cllc.2019.05.009
[3] Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial[J]. Lancet, 2016, 387(10027): 1540-1550. doi: 10.1016/S0140-6736(15)01281-7
[4] Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial[J]. Lancet Respir Med, 2021, 9(3): 305-314. doi: 10.1016/S2213-2600(20)30365-9
[5] Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial[J]. Lancet, 2017, 389(10066): 255-265. doi: 10.1016/S0140-6736(16)32517-X
[6] Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy[J]. Lancet Oncol, 2016, 17(12): e542-e551. doi: 10.1016/S1470-2045(16)30406-5
[7] Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy[J]. Clin Cancer Res, 2014, 20(19): 5064-5074. doi: 10.1158/1078-0432.CCR-13-3271
[8] Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy[J]. Nat Rev Cancer, 2016, 16(5): 275-287. doi: 10.1038/nrc.2016.36
[9] An HJ, Chon HJ, Kim C. Peripheral Blood-Based Biomarkers for Immune Checkpoint Inhibitors[J]. Int J Mol Sci, 2021, 22(17): 9414. doi: 10.3390/ijms22179414
[10] Kim KH, Kim CG, Shin EC. Peripheral blood immune cell-based biomarkers in anti-PD-1/PD-L1 therapy[J]. Immune Netw, 2020, 20(1): e8. doi: 10.4110/in.2020.20.e8
[11] Griffiths JI, Wallet P, Pflieger LT, et al. Circulating immune cell phenotype dynamics reflect the strength of tumor-immune cell interactions in patients during immunotherapy[J]. Proc Natl Acad Sci U S A, 2020, 117(27): 16072-16082. doi: 10.1073/pnas.1918937117
[12] Araujo B de Lima V, Hansen M, Spanggaard I, et al. Immune Cell Profiling of Peripheral Blood as Signature for Response During Checkpoint Inhibition Across Cancer Types[J]. Front Oncol, 2021, 11: 558248. doi: 10.3389/fonc.2021.558248
[13] Borst J, Ahrends T, Babala N, et al. CD4(+) T cell help in cancer immunology and immunotherapy[J]. Nat Rev Immunol, 2018, 18(10): 635-647. doi: 10.1038/s41577-018-0044-0
[14] Corthay A, Skovseth DK, Lundin KU, et al. Primary antitumor immune response mediated by CD4+ T cells[J]. Immunity, 2005, 22(3): 371-383. doi: 10.1016/j.immuni.2005.02.003
[15] Soyano AE, Dholaria B, Marin-Acevedo JA, et al. Peripheral blood biomarkers correlate with outcomes in advanced non-small cell lung Cancer patients treated with anti-PD-1 antibodies[J]. J Immunother Cancer, 2018, 6(1): 129. doi: 10.1186/s40425-018-0447-2
[16] Ottonello S, Genova C, Cossu I, et al. Association Between Response to Nivolumab Treatment and Peripheral Blood Lymphocyte Subsets in Patients With Non-small Cell Lung Cancer[J]. Front Immunol, 2020, 11: 125. doi: 10.3389/fimmu.2020.00125
[17] Nabet BY, Esfahani MS, Moding EJ, et al. Noninvasive Early Identification of Therapeutic Benefit from Immune Checkpoint Inhibition[J]. Cell, 2020, 183(2): 363-376, e313. doi: 10.1016/j.cell.2020.09.001
[18] Ivanova EA, Orekhov AN. T Helper Lymphocyte Subsets and Plasticity in Autoimmunity and Cancer: An Overview[J]. Biomed Res Int, 2015, 2015: 327470.
[19] Chen X, Liu Q, Xiang AP. CD8+CD28- T cells: not only age-related cells but a subset of regulatory T cells[J]. Cell Mol Immunol, 2018, 15(8): 734-736. doi: 10.1038/cmi.2017.153
[20] Hoesli R, Birkeland AC, Rosko AJ, et al. Proportion of CD4 and CD8 tumor infiltrating lymphocytes predicts survival in persistent/recurrent laryngeal squamous cell carcinoma[J]. Oral Oncol, 2018, 77: 83-89. doi: 10.1016/j.oraloncology.2017.12.003
[21] Liang X, Wei Z. Effect of Sintilimab combined with Chemotherapy on Tumor Markers and Immune Function of advanced non-small cell lung cancer[J]. Pak J Med Sci, 2021, 37(4): 1063-1068.
[22] 王芸, 王郁阳, 姜曼, 等. 帕博利珠单抗对晚期非小细胞肺癌患者T淋巴细胞亚群的影响及疗效观测[J]. 中国肺癌杂志, 2021, 24(3): 182-187. [Wang Y, Wang YY, Jiang M, et al. Effect of Pembrolizumab on T Lymphocyte Subsets in Patients with Advanced Non-small Cell Lung Cancer and Its Therapeutic Effect[J]. Zhongguo Fei Ai Za Zhi, 2021, 24(3): 182-187] Wang Y, Wang YY, Jiang M, et al. Effect of Pembrolizumab on T Lymphocyte Subsets in Patients with Advanced Non-small Cell Lung Cancer and Its Therapeutic Effect[J]. Zhongguo Fei Ai Za Zhi, 2021, 24(3): 182-187
[23] Rutkowski J, Cyman M, Slebioda T, et al. Evaluation of peripheral blood T lymphocyte surface activation markers and transcription factors in patients with early stage non-small cell lung cancer[J]. Cell Immunol, 2017, 322: 26-33. doi: 10.1016/j.cellimm.2017.09.007
[24] Li P, Qin P, Fu X, et al. Associations between peripheral blood lymphocyte subsets and clinical outcomes in patients with lung cancer treated with immune checkpoint inhibitor[J]. Ann Palliat Med, 2021, 10(3): 3039-3049. doi: 10.1186/s12885-022-09628-8
[25] Liu C, Wang Y, Li S, et al. Early change in peripheral CD4(+) T cells associated with clinical outcomes of immunotherapy in gastrointestinal cancer[J]. Immunotherapy, 2021, 13(1): 55-66. doi: 10.2217/imt-2020-0068
[26] Jansen CS, Prokhnevska N, Master VA, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells[J]. Nature, 2019, 576(7787): 465-470. doi: 10.1038/s41586-019-1836-5
-
期刊类型引用(2)
1. 诸琴红,盛瑜烈,王宝囡. 外周血CD8~+T淋巴细胞对Ⅲ期结直肠癌术后辅助化疗预后的影响. 浙江创伤外科. 2024(08): 1530-1533 .  百度学术
百度学术
2. 杨军炎,顾小丽. 胸腺法新联合DP方案化疗对非小细胞肺癌患者免疫功能及血清CEA和STK1水平的影响. 黑龙江医药. 2024(06): 1403-1406 .  百度学术
百度学术
其他类型引用(0)



 下载:
下载: