Use of Thread-hanging Microwave Antenna to Assist in Precise Puncture of Lung Nodule and Influencing Factors
-
摘要:目的
探讨挂线法辅助微波针肺结节精准穿刺方法,分析术中影响穿刺结果的若干现象。
方法收集107例恶性孤立性肺结节患者临床资料,其中原发性86例,转移性21例。肺结节CT轴状位长径平均(13.6±6)mm。采用挂线法辅助微波针肺结节穿刺。术中无退针,且插入结节中心区域为穿刺成功。记录并分析肺结节穿刺成功率、穿刺并发症和影响穿刺结果的针体裹挟、针体滑脱、针尖推顶、肺结节坠积、结节径向形变、结节掩蔽和针尖径向调针距离等若干现象发生率。
结果穿刺成功101例(94.4%),失败6例(5.6%)。气胸23例(21.5%),肺内出血19例(17.8%)。发生针体裹挟9例(8.4%),平均裹挟距离1.2±0.5 cm;针体滑脱6例(5.6%),平均针尖移动距离0.8±0.3 cm;针尖推顶19例(17.8%),平均推顶距离1.6±0.7 cm;肺结节坠积15例(14.0%),平均移动距离0.9±0.5 cm;结节径向形变14例(13.1%),平均牵拉长度增加0.5±0.3 cm;结节掩蔽5例(4.7%);针尖径向平均调针距离0.7±0.4 cm。
结论挂线法辅助微波针肺结节穿刺精度较高。术中应注意并处理影响穿刺结果的现象。
Abstract:ObjectiveTo explore a precise method with a microwave antenna for puncture of pulmonary nodules and analyze phenomena that affect the puncture results.
MethodsClinical data of 107 cases with solitary malignant pulmonary nodules were collected, and the mean length of pulmonary nodules was 13.6±0.6 mm in CT axial position. A thread-hanging method was used to assist the puncture of pulmonary nodules. The procedure was successful when the needle was not withdrawn and inserted into the central region of the nodule. The success rate and complications of the pulmonary procedure were recorded. The incidence of the following phenomena were also documented: needle coercing, needle slipping, needle tip pushing, pulmonary nodule prolapsing, radial nodule deformation, nodular masking, and radial movement distance of needle tip.
ResultsIn all of 107 cases evaluated, the antenna puncture was successful in 101 cases (94.4%) but failed in 6 cases (5.6%). Pneumothorax and pulmonary hemorrhage occurred in 23 (21.5%) and 19 cases (17.8%), respectively. The following phenomena occurred: needle coercing in 9 cases (8.4%), needle slipping in 6 cases (5.6%), needle tip pushing in 19 cases (17.8%), pulmonary nodule prolapsing in 15 cases (14%), radial nodule deformation in 14 cases (13.1%), and nodular masking in 5 cases (4.7%). The mean radial adjusting distance of needle tip was 0.7±0.4 cm.
ConclusionThe thread-hanging method can assist in the accurate puncture of microwave antenna for pulmonary nodules. We should focus and deal with phenomena that may occur and affect the result of puncture.
-
Key words:
- CT-guided /
- Microwave antenna /
- Accurate puncture /
- Pulmonary nodules
-
0 引言
经皮微波消融术是恶性肺结节主要治疗方法之一[1],精准穿刺是确保消融效果的重要基础。尽管业内探索多种辅助精准穿刺方法和技术[2-15],但是迄今,CT引导下肺结节精准穿刺依然是介入医师和影像医师关注的焦点[16-17]。为提高穿刺精度,我们收集恶性肺结节107例,采用挂线法辅助微波针肺结节穿刺,并分析术中影响穿刺结果的若干现象,旨在提高肺结节穿刺成功率和安全性,现将方法和结果报道如下。
1 资料与方法
1.1 一般资料
收集石家庄市中医院2019年2月—2023年3月经细胞学和(或)组织病理学确诊的107例孤立性恶性肺结节患者,其中男71例,女36例。年龄21~86岁,中位年龄56.9岁。原发性肺癌86例,转移性21例。CT肺结节轴状位长径5~25 mm,平均13.6±6 mm,其中5~10 mm 23例,11~20 mm 63例,21~25 mm 21例。实性肺结节(solid pulmonary nodules, sPN)59例,混合性磨玻璃结节(mixed ground-glass nodules, mGGN)32例,纯磨玻璃结节(pure ground-glass nodules, pGGN)16例。肺结节距胸膜≤1 cm 21例,1~5 cm 59例,>5 cm 27例。43例患者有基础性肺疾病,其中慢性阻塞性肺疾病(chronic obstructive pulmonary disease, COPD)14例、哮喘11例、肺纤维化9例、肺结核6例和矽肺3例。
1.2 设备、器械和术前准备
美国GE 64排螺旋CT,南京康友微波能应用研究所KY-2200型微波消融治疗仪,微波消融针(微波针)KY-2450B,白色棉线1条,长100 cm,经高温、高压蒸汽消毒后备用,血管钳,若干纱布垫,手术铺巾,穿刺包,监护、吸氧等设备。患者术前进行血常规、凝血功能、心肺功能及胸部增强CT检查,排除肺穿刺消融禁忌证。嘱患者平静呼吸训练。告知家属或患者操作风险和注意事项,并签署知情同意书。本研究已通过石家庄市中医院伦理委员会审查。
1.3 方法
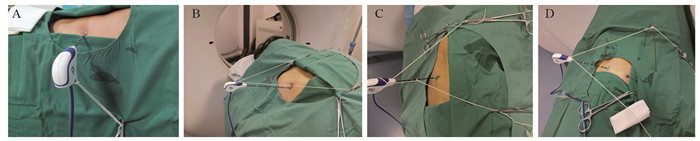
患者取多种体位,以其舒适、方便术者操作为目的。CT扫描参数:层厚1.25 mm,层间距1.25 mm。将定位栅放置在拟穿刺体表部位,平静呼吸状态下确定进针点、进针方向和深度。胸壁消毒、铺巾和麻醉后,经进针点、沿进针方向将消融针插入胸壁。取长100 cm白色细丝线一条,其中间固定在消融微波针针杆或尾部,两端甩出线头50 cm,根据肺CT显示针尖与结节的空间位置,牵拉两端丝线的方向和张力,调整针尖方向(调针),并用血管钳将丝线固定在铺巾表面,见图 1。
当针尖指向结节时,向前推送针体(进针),即调针、进针序贯进行(序贯穿刺模式)。随后,CT扫描核实结节与针尖位置,如果针尖方向偏离肺结节,则再次调整丝线的牵拉方向和张力,当针尖指向结节后,向前推送针体,直至针尖插入结节中心部位,启动消融过程,见图 2。本操作由两位有18年肺穿刺经验的医师独立完成。
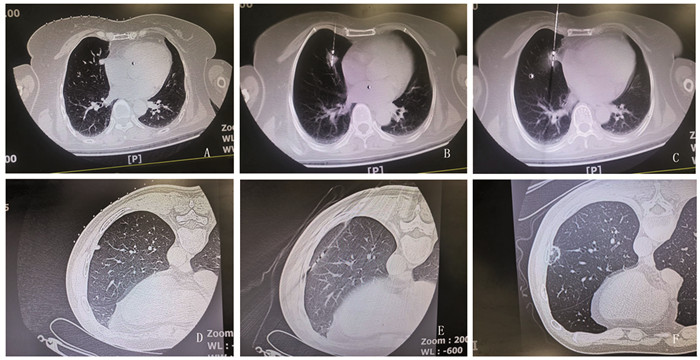
![]() 图 2 微波针穿刺肺结节CT示意图Figure 2 CT operation diagram of using microwave needle in puncturing pulmonary nodulesA: the mediastinal subpleural nodule of middle lobe of the right lung is 1.0 cm in length; B: the tip of the ablation needle was inserted into the pulmonary nodules, and the nodules were radially adjusted to remove 1 cm from the pleura to initiate ablation; C: euring nodular ablation, needle tip slip occurred due to cough, and the tip moved 1 cm forward beyond the anterior edge of the nodules; D: the subpleural nodule of lower lobe of the right lung has a diameter of 0.5 cm; E: needle tip pushing nodules forward 2.5 cm; F: CT reexamination 2 days after ablation; A-C: an 87-year-old female with mediastinal subpleural lung adenocarcinoma in middle lobe of the right lung; D-F: a 59-year-old man with subpleural metastases in inferior lobe of the left lung 4 years after nephrectomy of renal carcinoma.
图 2 微波针穿刺肺结节CT示意图Figure 2 CT operation diagram of using microwave needle in puncturing pulmonary nodulesA: the mediastinal subpleural nodule of middle lobe of the right lung is 1.0 cm in length; B: the tip of the ablation needle was inserted into the pulmonary nodules, and the nodules were radially adjusted to remove 1 cm from the pleura to initiate ablation; C: euring nodular ablation, needle tip slip occurred due to cough, and the tip moved 1 cm forward beyond the anterior edge of the nodules; D: the subpleural nodule of lower lobe of the right lung has a diameter of 0.5 cm; E: needle tip pushing nodules forward 2.5 cm; F: CT reexamination 2 days after ablation; A-C: an 87-year-old female with mediastinal subpleural lung adenocarcinoma in middle lobe of the right lung; D-F: a 59-year-old man with subpleural metastases in inferior lobe of the left lung 4 years after nephrectomy of renal carcinoma.1.4 观察内容
穿刺成功:穿刺过程中无退针,且针尖插入结节中心区域(3环靶区中心1/3区域),反之为失败。记录穿刺成功率、穿刺并发症,以及术中出现、并影响穿刺结果的若干现象:(1)针体裹挟现象:进入肺内的针体受到针道周围组织夹持,在一定进针范围内针体与周围组织同时、同向和等距离向前移动(≥0.5 cm),针尖在肺内没有实质性向前移动;(2)针体滑脱现象:进针深度明显超出预定进针深度(≥0.5 cm);(3)针尖推顶现象:进针时针尖推顶结节向前移位(≥0.5 cm),但针尖未能进入结节或预定结节位置;(4)肺结节坠积现象:受自身重力作用,肺结节呈现下沉移位(≥0.5 cm);(5)结节径向形变现象:进入结节的针尖径向移动时,结节受到针尖推、拉作用,引起结节发生形变,受到针尖推顶一侧的结节组织压缩,而受到针尖牵拉一侧的结节拉长≥0.3 cm;(6)结节掩蔽现象:穿刺过程中,肺结节(大小、边缘、形态、密度、或位置)与其周围肺组织结构分辨不清;(7)针尖径向调针距离:当径向力作用针尾时,针尖在肺内发生相反方向移动。
2 结果
消融微波针穿刺成功101例(94.4%),失败6例(5.6%),其中退针2例,针尖插入肺结节非中心区域4例(3环靶区中、外2/3区域)。气胸23例(21.5%),肺内出血19例(17.8%),均为少量。术中发生针体裹挟现象9例(8.4%),裹挟距离0.5~2.5 cm(平均距离1.2±0.5 cm);针体滑脱现象6例(5.6%),针尖向前移动超出预定进针深度0.5~2.3 cm(平均距离0.8±0.3 cm);针尖推顶现象19例(17.8%),推顶距离0.5~6.5 cm(平均距离1.6±0.7 cm);肺结节坠积现象15例(14.0%),体位改变后肺结节移动距离0.5~2.4 cm(平均距离0.9±0.5 cm);结节径向形变现象14例(13.1%),针尖牵拉一侧的结节长度增加0.3~1.2 cm(平均距离0.5±0.3 cm)。结节掩蔽现象5例(4.7%);针尖径向调针距离0.3~3.6 cm(平均0.7±0.4 cm)。
3 讨论
目前CT引导下肺结节徒手穿刺依然广泛应用[18],若提高穿刺精度需要术者长期经验积累[19-20],因此该穿刺技术的推广和应用受到限制。现行CT引导下,徒手肺结节穿刺属于盲穿,不可避免偏离预定方向,于是术者在消融针尾施加一个径向外力,矫正针尖方向,结果针道周围的组织发生形变,内应力产生,其力的大小与针尾外力大小相等、方向相反,这种由径向调针产生的内应力称为静态内应力。当作用于针尾的外力消失后,内应力释放,针尖恢复原位,因此有效调针不仅需要调整针向,同时还需要向前推送针体,即调针-进针同步进行(同步穿刺模式),于是针尖产生一个反向内应力,前后内应力综合作用结果决定针尖方向。因此术者需依赖经验、多次调针、进针、甚至退针、才能使针尖插入肺结节预设部位,穿刺精度受到限制。
呼吸时胸廓与肺反向运动,作用于针体的内应力大小和方向随呼吸实时发生改变—即动态内应力,导致针体摆动。吸气时胸骨、肋骨上举,胸廓扩张,致使肺组织前后径和横径扩大(横向),这种肺横向移动产生的内应力对于横向走行的针体作用较小。然而,胸骨、肋骨上举产生的纵向内应力对于横向走行的针体作用较大,导致针体纵向摆动,摆动幅度随胸廓部位下移而逐渐增加,Chen等报道,平静呼吸时85例肺结节移动距离平均1.76 cm,且与结节大小和胸膜距离无关[21]。同样,正常成人平静呼吸时,膈肌移动幅度可达2~4 cm,由此而产生的纵向内应力作用于针体,使针体摆动进一步强化,这种摆动幅度在近膈肌处最为明显。针体摆动的中心轴位于肋间隙或附近,由于胸壁组织密度高、弹性差,而肺组织密度低弹性高,针尖方向主要取决于胸壁内应力,意味着若要对抗胸壁内应力,需要肺内更大的调针幅度和更长的进针距离。同时,鉴于杠杆效应,导致肺内针尖纵向移动距离与肺结节不完全一致,特别当针体胸壁内,或肺内走行距离较长时,穿刺精度进一步受到限制。
采用微波针尾挂线方法,应用序贯穿刺模式可提高肺结节穿刺精度。由于丝线两端固定在铺巾表面,一个大小、方向恒定的外力始终作用于针尾,使得静、动态内应力无法释放,从而针体呼吸摆动消失,针体周围肺组织和肺结节受到制动,使得针尖位置在CT扫描时与穿刺时高度一致。另外,序贯穿刺模式将穿刺过程分解成调针、进针两个过程,调针时术者不再顾忌内应力影响,可以根据CT显示的针尖与肺结节位置,通过改变丝线两端牵拉方向和张力,调整针尖方向,当针尖指向结节中心时,再向前推送针体。至此,调针和进针有了客观、量化、稳定参照物,减少了对经验的依赖,从而提高穿刺精度。Tsai等[22]报道采用无菌孔巾作为支托、调整针尖方向,辅助同轴针肺结节穿刺,虽然文中未提及内应力概念,确是最早应用序贯穿刺模式的范例,遗憾的是该方法调针、控针效率低下。
本研究应用挂线方法包括:单线针尾挂线方法(图 1A):当针体呼吸摆动小或无时,适宜应用本挂线法调整针尖方向;双线(针尾、针杆)挂线方法(图 1B~C):(1)当针体呼吸摆动较大时,适宜应用双线挂线对抗针体摆动,特别丝线牵拉方向与针体摆动方向一致时;(2)当消融针尖插入胸壁或表浅时,体外针体受重力和呼吸作用发生偏移,引起针尖移位,适宜应用双线(甚至三线)对抗针体受重力和呼吸作用发生偏移,稳定针体和针尖方向;(3)当针体与CT扫描床平行或近乎平行方向进针时,针尾受重力作用下垂,适宜应用双线保持预设针体位置和方向(图 1B~D);支架辅助针尾挂线方法(图 1D):主要用于针体与CT扫描床平行或近乎平行进针时,或与胸膜切线平行路径进针时(图 2D~E),这时牵拉丝线与消融针成角过小,丝线牵拉力矩小,若要调整针尖方向需要加大拉力,丝线张力增加容易拖拽孔巾移位,甚至引起针尖非意愿性向前移动。单或双支架改变了力的作用点,增加了丝线牵拉力矩,血管钳丝线固定点受力明显减小。
本研究消融针穿刺成功101例(94.4%),显示挂线法辅助穿刺具有较高成功率。当针尖距离结节≤0.5 cm时,应精细调针,谨慎进针。尤其针尖插入结节表层并偏离中心区域时,对于mGGN和pGGN仍可尝试结节内调针,将针尖插入结节中心区域。反之部分sPN结节内调针困难,常需退针到结节边缘,重新调整针向、进针;胸膜下结节宜采用与胸膜切线平行路径进针,尽可能肺内调针距离≥2 cm,此时消融针与胸壁夹角可能很小,宜采用支架辅助挂线法调整针向(图 1D、图 2D~E);当肺结节前缘或侧缘毗邻胸膜、血管等重要结构时,先将针尖插入结节中心,采用挂线法径向拨动结节脱离前缘或侧壁重要结构,随后针尖贯穿结节,保持丝线张力和安全距离后,启动消融过程(图 2A~C)。针尖径向调针是实现结节精准穿刺的重要方法(图 2A~B),调针距离受多种因素影响:如针杆硬度、长度、胸壁及肺内走行距离、针尾受力大小、肋间隙宽度、针杆与肋骨上下缘距离、基础肺疾病和针尖肺内部位等。穿刺失败6例(5.6%),失败原因与胸壁厚、结节小、磨玻璃样结节、毗邻胸膜或膈肌结节、呼吸幅度大以及针尖推顶、针体滑脱和掩蔽现象有关。其中退针2例,针尖插入肺结节非中心区域4例(3环靶区中、外2/3区域)。
气胸和肺内出血是经皮肺穿刺常见并发症[23]。本研究发生气胸23例(21.5%),多出现在消融针退出胸壁后,肺内出血19例(17.8%),其中针道出血16例、消融区出血2例、针道和消融区均出血1例,均为少量。减少气胸发生的关键在于预设穿刺路径时避开肺气肿、肺大泡或减少胸膜损伤。对于穿刺路径中(横向、纵向)血管结构,可通过挂线法,调整针尖方向,避开血管后进针,当针尖避开或跨越血管后,回调丝线使针尖恢复原有方向后进针。
本研究中针体裹挟现象多见于胸壁厚、背部入路、肌肉紧张或步进距离短、速度慢患者;针尖推顶(推移)现象多见于结节小、密度高,位于肺尖,肺底或肺周边部位,或步进距离短、速度慢时(图 2D、E);针体滑脱现象多见于针体裹挟现象、结节顶推现象、快速进针、消融中或咳嗽时(图 2B、C);肺结节坠积现象多见于实性、较大或位于肺周边部位肺结节,坠积距离与体位有关,亦与时间有关(即时坠积,延时坠积);结节径向形变现象见于径向调针结节,结节形变主要与结节大小、密度、部位、肺部基础性疾病,以及径向受力大小相关;结节掩蔽现象多发生在小结节、mGGN、pGGN或肺(组织或血液)坠积部位,肺出血、针伪影或结节推顶、形变亦可出现掩蔽现象。
目前,上述若干现象鲜有文献报道,其产生原因、机制、临床意义,以及术前预判、术中防范和术中处理等亟待探索。
综上所述,应用挂线法辅助肺结节穿刺可提高穿刺成功率和降低并发症。应注意并处理术中可能出现并影响穿刺结果的现象。但本研究样本量小,属于单中心、回顾性非对照研究,期待更多深入研究。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:金辉:研究构思与设计,论文撰写谭捷:研究构思与设计,实操指导,论文撰写、修改宋珊、王英虎、马丽华:研究实施,资料收集,文献检索与论文撰写 -
[1] Ye X, Fan W, Wang Z, et al. Expert consensus on thermal ablation therapy of pulmonary subsolid nodules (2021 Edition)[J]. J Cancer Res Ther, 2021, 17(5): 1141-1156. doi: 10.4103/jcrt.jcrt_1485_21
[2] Teng J, Xu J, Jiao J, et al. Radiofrequency ablation of synchronous multiple primary lung cancer assisted by a magnetic navigation system: a case report[J]. Ann Palliat Med, 2020, 9(2): 478-482. doi: 10.21037/apm.2020.02.21
[3] Wu Q, Cao B, Zheng Y, et al. Feasibility and safety of fine positioning needle-mediated breathing control in CT-guided percutaneous puncture of small lung/liver nodules adjacent to diaphragm[J]. Sci Rep, 2021, 11(1): 3411. doi: 10.1038/s41598-021-83036-z
[4] 宋桂松, 邱桐, 玄云鹏, 等. 电磁导航支气管镜矢量定位法在肺小结节手术中的应用[J]. 中国肺癌杂志, 2019, 22(11): 709-713. doi: 10.3779/j.issn.1009-3419.2019.11.05 Song GS, Qiu T, Xuan YP, et al. Clinical Application of Vectorial Localization of Peripheral Pulmonary NodulesGuided by Electromagnetic Navigation Bronchoscopy in Thoracic Surgery[J]. Zhongguo Fei Ai Za Zhi, 2019, 22(11): 709-713. doi: 10.3779/j.issn.1009-3419.2019.11.05
[5] Tsai SC, Wu TC, Lai YL, et al. Preoperative computed tomography-guided pulmonary nodule localization augmented by laser angle guide assembly[J]. J Thorac Dis, 2019, 11(11): 4682-4692. doi: 10.21037/jtd.2019.10.60
[6] Fu R, Zhang C, Zhang T, et al. A three-dimensional printing navigational template combined with mixed reality technique for localizing pulmonary nodules[J]. Interact Cardiovasc Thorac Surg, 2021, 32(4): 552-559. doi: 10.1093/icvts/ivaa300
[7] Fior D, Vacirca F, Leni D, et al. Virtual Guidance of Percutaneous Transthoracic Needle Biopsy with C-Arm Cone-Beam CT: Diagnostic Accuracy, Risk Factors and Effective Radiation Dose[J]. Cardiovasc Intervent Radiol, 2019, 42(5): 712-719. doi: 10.1007/s00270-019-02163-3
[8] Taralli S, Scolozzi V, Foti M, et al. 18F-FDG PET/CT diagnostic performance in solitary and multiple pulmonary nodules detected in patients with previous cancer history: reports of 182 nodules[J]. Eur J Nucl Med Mol Imaging, 2019, 46(2): 429-436. doi: 10.1007/s00259-018-4226-6
[9] Fielding DIK, Bashirzadeh F, Son JH, et al. First human use of a new robotic-assisted fiber optic sensing navigation system for small peripheral pulmonary nodules[J]. Respiration, 2019, 98(2): 142-150. doi: 10.1159/000498951
[10] Ji Z, Wang G, Chen B, et al. Clinical application of planar puncture template-assisted computed tomography-guided percutaneous biopsy for small pulmonary nodules[J]. J Cancer Res Ther, 2018, 14(7): 1632-1637. doi: 10.4103/jcrt.JCRT_1017_17
[11] Casal RF, Sarkiss M, Jones AK, et al. Cone beam computed tomography-guided thin / ultrathin bronchoscopy for diagnosis of peripheral lung nodules: a prospective pilot study[J]. J Thorac Dis, 2018, 10(12): 6950-6959. doi: 10.21037/jtd.2018.11.21
[12] Jeon MC, Kim JO, Jung SS, et al. CT-Guided Percutaneous Transthoracic Needle Biopsy Using the Additional Laser Guidance System by a Pulmonologist with 2 Years of Experience in CT-Guided Percutaneous Transthoracic Needle Biopsy[J]. Tuberc Respir Dis, 2018, 81(4): 330-338. doi: 10.4046/trd.2017.0123
[13] Fu YF, Fi GC, Cao W, et al. Computed Tomography Fluoroscopy-Guided Versus Conventional Computed Tomography-Guided Lung Biopsy: A Systematic Review and Meta-analysis[J]. J Comput Assist Tomogr, 2020, 44(4): 571-577. doi: 10.1097/RCT.0000000000001044
[14] Iannelli G, Caivano R, Villonio A, et al. Percutaneous Computed Tomography-Guided Lung Biopsies using a Virtual Navigation Guidance: Our Experience[J]. Cancer Invest, 2018, 36(6): 349-355. doi: 10.1080/07357907.2018.1498877
[15] Heerink WJ, Ruiter SJS, Pennings JP, et al. Robotic versus Freehand Needle Positioning in CT-guided Ablation of Liver Tumors: A Randomized Controlled Trial[J]. Radiology, 2019, 290(3): 826-832. doi: 10.1148/radiol.2018181698
[16] Lee KH, Lim KY, Suh YJ, et al. Diagnosic Accuracy of Percuaneous transthoracic Needle Lung Biopsies: A Multicenter Study[J]. Korean J Radiol, 2019, 20(8): 1300-1310. doi: 10.3348/kjr.2019.0189
[17] Huang MD, Weng HH, Hsu SL, et al. Accuracy and complications of CT-guided pulmonary core biopsy in small nodules: a single-center experience[J]. Cancer Imaging, 2019, 19(1): 51. doi: 10.1186/s40644-019-0240-6
[18] Sidoff L, Dupuy DE. Clinical experiences with microwave thermal ablatiom of lung malignancies[J]. Int J Hyperthermia, 2017, 33(1): 25-33. doi: 10.1080/02656736.2016.1204630
[19] Bayrak BY, Paksoy N, Vural Ç, et al. Diagnostic utility of fine needle aspiration cytology and core biopsy histopathology with or without immunohistochemical staining in the subtyping of the non-small cell lung carcinomas: Experience from an academic centre in Turkey[J]. Cytopathology, 2021, 32(3): 331-337 doi: 10.1111/cyt.12937
[20] Heerink WJ, Ruiter SJS, Pennings JP, et al. Robotic versus freehand needle positioning in CT-guided ablation of liver tumors: A randomized controlled trial[J]. Radiology, 2019, 290(3): 826-832. doi: 10.1148/radiol.2018181698
[21] Chen A, Pastis N, Furukawa B, et al. The effect of respiratory motion on pulmonary nodule location during electromagnetic navigation bronchoscopy[J]. Chest, 2015, 147(5): 1275-1281. doi: 10.1378/chest.14-1425
[22] Tsai IC, Tsai WL, Chen MC, et al. CT-guided core biopsy of lung lesions: a primer[J]. AJR Am J Roentgenol, 2009, 193(5): 1228-1235. doi: 10.2214/AJR.08.2113
[23] Guo Z, Shi H, Li W, et al. Chinese multidisciplinary expert consensus: Guidelines on percutaneous transthoracic needle biopsy[J]. Thorac Cancer, 2018, 9(11): 1530-1543. doi: 10.1111/1759-7714.12849



 下载:
下载: