Short-term Outcomes and Long-term Survival Outcomes of Elderly Patients (Over 80 Years of Age) with Colorectal Cancer Who Received Laparoscopic Versus Open Surgery
-
摘要:目的
探讨80岁以上高龄结直肠癌患者行腹腔镜与开腹手术的近期疗效与远期预后。
方法纳入行根治性手术的80岁以上313例高龄结直肠癌患者, 其中开腹组143例、腹腔镜组170例。采用倾向得分匹配平衡两组患者基线资料。Kaplan-Meier绘制生存曲线, Log rank法进行预后比较。Cox比例风险模型分析各因素对总体生存率(OS)和无瘤生存率(DFS)的影响。
结果匹配后, 两组各纳入93例患者。腹腔镜组患者平均术中出血量、术后总体并发症与Ⅰ~Ⅱ级并发症发生率低于开腹组(均P < 0.05)、术后首次排气时间、经口进食时间与住院时间均明显短于开腹组患者(均P < 0.05)、平均淋巴结清扫数量显著多于开腹组(P=0.030)。两组患者有着相似的5年OS (P=0.594)与DFS (P=0.295)。多因素Cox预后分析结果发现CEA水平 > 5 ng/ml、病理TNM分期Ⅲ期与神经侵犯是影响患者不良OS与DFS的独立危险因素。
结论与开腹手术相比, 腹腔镜手术可以为80岁以上高龄结直肠癌患者提供更好的短期治疗效果与相似的远期预后。
Abstract:ObjectiveTo examine short-term outcomes and long-term survival of elderly patients (aged over 80 years) with colorectal cancer who received laparoscopic versus open surgery.
MethodsA total of 313 patients over 80 years old with colorectal cancer who underwent radical surgery were included.According to the surgical method, all patients were divided into open-surgery group (n=143) and laparoscopic surgery group (n=170).Baseline data were balanced between the two groups by using propensity score matching.Kaplan-Meier was used to draw the survival curve, and survival was compared by Log rank tests.Cox proportional risk model was used to analyze the effects of all factors on overall survival (OS) and disease-free survival (DFS).
ResultsAfter matching, 93 patients were included in each group.The mean intraoperative blood loss, the incidence of overall postoperative complications and gradeⅠ-Ⅱ complications in the laparoscopic surgery group were significantly lower than those in the open surgery group (all P < 0.05).The time to first flatus, the time to oral feeding, and postoperative hospital stays in the laparoscopic surgery group were significantly shorter than those in the open surgery group (all P < 0.05).The mean number of lymph-node dissection was also significantly higher in the laparoscopic surgery group than in the open surgery group (P=0.030).Patients in both groups had similar 5-year OS (P=0.594) and DFS (P=0.295).Multivariate Cox prognostic analysis showed that CEA level > 5 ng/ml, pathological TNM stage Ⅲ, and perineural invasion were independent risk factors for poor OS and DFS.
ConclusionCompared with open surgery, laparoscopic surgery can provide better short-term advantages and similar long-term outcomes for colorectal cancer patients over 80 years of age.
-
Key words:
- Colorectal cancer /
- Open surgery /
- Laparoscopic surgery /
- Short-term outcomes /
- Prognosis /
- Elderly patients
-
0 引言
结直肠癌的发病率与死亡率分别占所有恶性肿瘤的第三位与第四位[1]。根据世界癌症研究中心GLOBOCAN 2018数据库,中国65岁以上老年人群结直肠癌的发病率仍处于逐年上升趋势[2]。腹腔镜结直肠癌手术与开腹手术相比,具有切口小、恢复快、住院时间短等短期优势,在临床上得到了广泛应用[3-5]。既往研究证实了腹腔镜在老年结直肠癌患者中应用的安全性和短期优势[6-9],然而,这些研究纳入患者的中位年龄为65~75岁。对于80岁以上的高龄结直肠癌患者是否也能从腹腔镜手术中获益,目前仍存在争议[10-13]。因此,本研究拟采用倾向得分匹配(propensity score matching, PSM)方法来探讨80岁以上高龄患者行腹腔镜与开腹结直肠癌手术的近期疗效与远期预后。
1 资料与方法
1.1 资料来源
本研究为多中心回顾性研究。收集2007年1月—2020年12月在中国医学科学院肿瘤医院与新乡市中心医院行手术治疗的结直肠癌患者数据资料。纳入标准:(1)年龄≥80岁;(2)病理类型为腺癌;(3)根治性手术。排除标准:(1)因肠穿孔、梗阻行急诊手术;(2)合并肝、肺、骨等远处转移;(3)既往合并其他恶性肿瘤病史;(4)姑息性切除;(5)行新辅助治疗者。共313例患者纳入本研究。根据手术入路不同,将入组患者分为腹腔镜组(n=170)与开腹组(n=143)。本研究的开展与执行均通过各机构伦理委员会批准同意。所有入组患者均在术前签署知情同意书。
1.2 诊断与定义
本研究基于各中心电子病案系统记录收集患者的临床特征、围手术期数据、病理结果以及预后资料。所有患者术前均需完善体格检查、实验室检查、内镜活检、胸腹盆CT等。此外,所有患者还需常规完善心电图、超声心动图、肺功能检查、下肢深静脉B超等评估患者心血管情况。所有手术均由具有20年以上结直肠癌手术经验的外科医生完成。手术后出现以下情况需进入ICU进一步密切监测与治疗:(1)术中麻醉期间出现严重心率失常或心脏骤停;(2)术中血压升高,对症处理后收缩压仍 > 180 mmHg,或严重低血压对症处理后收缩压仍低于80 mmHg;(3)术前肺功能差,术中或术后出现SaO2 < 90%的严重低氧血症,需机械通气维持氧合;(4)手术复杂时间冗长,且术前合并严重心肺器质性病变,术中生命体征不平稳需要密切监护。采用美国癌症联合委员会(American Joint Committee on Cancer, AJCC)第八版进行TNM分期。术后并发症定义为影响患者术后正常恢复过程的事件,并根据ClavienDindo分级系统对患者并发症进行分级[14],Ⅲ~Ⅳ级为严重并发症。
1.3 生存分析方法
根治性手术后前3年,所有患者每3~6个月通过门诊或电话的形式进行随访。门诊随访时需完成的复查项目有:体格检测、实验室检查、肿瘤标志物(CEA和CA19-9)、胸腹盆CT等。手术3年后,患者每6~12个月进行一次随访,直至患者死亡或截至2022年12月31日。本研究终点为5年总体生存率(OS)与5年无瘤生存率(DFS)。
1.4 统计学方法
采用PSM方法以减少两组间患者基线资料差异,纳入的匹配协变量包括:年龄、性别、体质量指数、术前血红蛋白水平、术前白蛋白水平、美国麻醉医师协会分级、并发症、既往腹部手术史、原发肿瘤位置、临床TNM分期与术前CEA水平。
本研究采用SPSS24.0进行统计学分析。连续性变量通过t检验进行组间比较,并采用均数±标准差表示。分类变量采用卡方检验进行组间比较,并采用例数(%)进行表示。生存分析方面,本研究采用Kaplan-Meier绘制两组患者生存曲线,通过Log rank法进行预后生存比较。将单因素分析中有显著统计学差异的变量纳入多因素Cox回归模型分析,以风险比(Hazard ratio, HR)和95%CI评价各变量对预后的影响。P < 0.05为差异有统计学意义。
2 结果
2.1 患者基线临床病理资料
共313例患者纳入研究,其中275例来自中国医学科学院肿瘤医院,38例来自新乡市中心医院。匹配前,开腹组患者平均体质量指数(22.7 vs. 23.7 kg/m2, P=0.009)、血红蛋白水平(120.0 vs. 124.5 g/L, P=0.045)、白蛋白水平(37.2 vs. 40.2 g/L, P < 0.001)均低于腹腔镜组患者。此外,开腹组患者中美国麻醉医师协会(American Society of Anesthesiologists, ASA)分级为Ⅲ~Ⅳ级的患者比例显著高于腹腔镜组(63.6% vs. 34.7%, P < 0.001)。腹腔镜组患者术前存在并发症的患者比例显著高于开腹组患者(72.4% vs. 58.0%, P=0.008)。腹腔镜组患者原发肿瘤位于直肠的比例显著高于开腹组(50.0% vs. 35.7%, P=0.007)。
采用PSM平衡两组患者基线资料,使组间结果具有可比性,匹配后两组各有93例患者纳入研究。匹配后,两组患者基线临床病理资料均衡,差异无统计学意义(P > 0.05),见表 1。
表 1 匹配前、后开腹组与腹腔镜组老年患者临床病理特征(n(%))Table 1 Clinicopathological features of elderly patients in the open surgery and laparoscopic surgery groups before and after matching (n(%))
2.2 短期治疗结果
匹配后开腹组与腹腔镜组患者手术时间相似(155.1 vs. 161.2 min, P=0.464),但腹腔镜组术中预估出血量显著少于开腹组(50.9 vs. 108.1 ml, P < 0.001)。开腹组与腹腔镜组患者术中输血的比例差异无统计学意义(22.6% vs. 16.1%, P=0.265)。腹腔镜组中有1例(1.1%)患者因腹腔内广泛粘连而中转开腹手术。术后并发症方面,开腹组患者术后总体并发症(26.9% vs. 10.8%, P=0.005)与Ⅰ~Ⅱ级并发症发生率显著高于腹腔镜组患者(17.2% vs. 6.5%, P=0.023),而两组患者的Ⅲ~Ⅳ级严重并发症发生率差异无统计学意义(9.7% vs. 4.3%, P=0.150)。开腹组最常见的术后并发症为切口感染(9.7%),其次为肠梗阻(5.4%)、吻合口瘘(4.3%)、胃排空延迟(4.3%)。腹腔镜组最常见的并发症为吻合口瘘(2.2%)、肠梗阻(2.2%)和肺炎(2.2%)。术后恢复方面,腹腔镜组患者术后首次排气时间(4.5 vs. 5.5天, P=0.001)、经口进食时间(4.8 vs. 5.9天, P=0.003)与住院时间(9.6 vs. 12.2天, P < 0.001)均明显短于开腹组患者。围手术期两组患者均无死亡病例,见表 2。
表 2 匹配后开腹组与腹腔镜组老年患者围手术期结局Table 2 Perioperative outcomes of elderly patients in the open surgery and laparoscopic surgery groups after matching
2.3 病理结果
腹腔镜组患者平均淋巴结清扫数量显著多于开腹组(20.3 vs. 17.2枚, P=0.030)。两组患者肿瘤直径、神经侵犯、脉管瘤栓、环周切缘、肿瘤分化程度与病理TNM分期方面差异无统计学意义(P > 0.05),见表 3。
表 3 匹配后开腹组与腹腔镜组老年患者病理结果(n(%))Table 3 Pathologic outcomes of elderly patients in the open surgery and laparoscopic surgery groups after matching (n(%))
2.4 生存预后
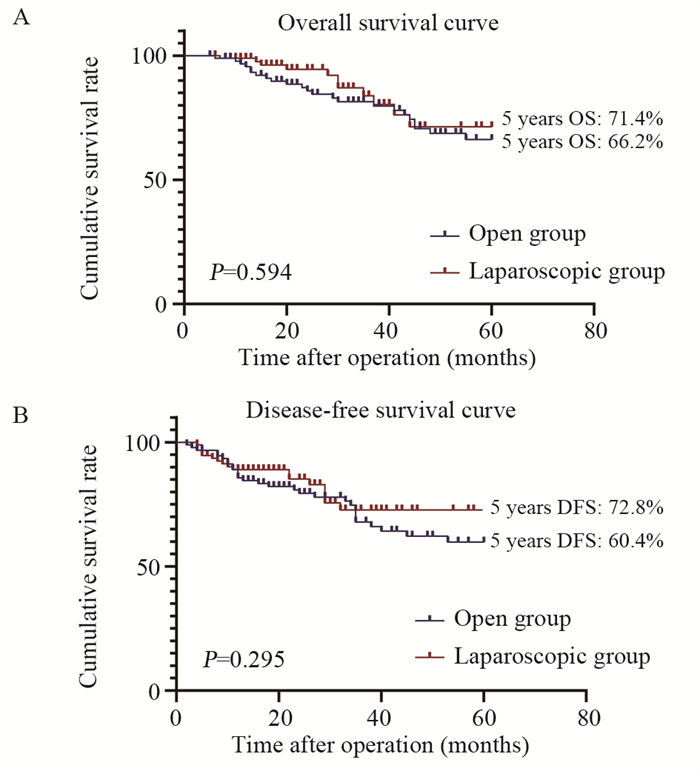
匹配后,腹腔镜组患者中位随访时间为57个月,开腹组患者中位随访时间为60个月。两组患者的5年OS(71.4% vs. 66.2%, P=0.594)与DFS(72.8% vs. 60.4%, P=0.295)差异无统计学意义,见图 1。
对186例患者进行预后分析,单因素分析发现CEA水平 > 5 ng/ml、N分期、病理TNM分期、神经侵犯与脉管瘤栓显著影响患者OS与DFS(P < 0.05)。将单因素分析中有统计学差异的变量纳入多因素Cox回归预测模型,结果发现CEA水平 > 5 ng/ml(HR: 1.77, 95%CI: 1.29~4.15, P=0.038)、病理TNM分期Ⅲ期(HR: 9.67, 95% CI: 3.18~79.30, P=0.012)与神经侵犯(HR: 1.57, 95%CI: 1.15~3.21, P=0.041)是影响不良OS的独立危险因素。同样,CEA水平 > 5 ng/ml(HR: 2.32, 95%CI: 1.26~4.98, P=0.022)、病理TNM分期Ⅲ期(HR: 9.82; 95%CI: 3.15~83.55; P=0.002)与神经侵犯(HR: 2.09, 95%CI: 1.59~5.32, P=0.020)亦是导致不良DFS的独立危险因素,见表 4。
表 4 匹配后老年患者总体生存率与无瘤生存率的单因素与多因素Cox回归分析Table 4 Univariate and multivariate Cox regression analyses of overall survival and disease-free survival in elderly patients after matching
3 讨论
80岁以上高龄结直肠癌患者具有特殊的临床病理特征及预后,且腹腔镜手术应用于高龄结直肠癌患者可行性与安全性仍亟待证实。本研究发现,在80岁以上的高龄结直肠癌患者中,腹腔镜手术的短期治疗效果优于开腹手术,在长期生存预后方面无显著差异。此外,CEA水平、TNM分期、神经侵犯是80岁以上高龄结直肠癌患者根治术后OS和DFS的可靠预测指标。
先前研究结果表明,老年结直肠癌患者也可以通过腹腔镜手术获得较好的短期疗效[6-13]。本研究结果同样发现高龄结直肠癌患者行腹腔镜手术可以明显减少术中出血量(50.9 vs. 108.1 ml, P < 0.001)和降低术后并发症发生率(10.8% vs. 26.9%, P=0.005),尤其是Ⅰ~Ⅱ级并发症发生率(17.2% vs. 6.5%, P=0.023)。术中失血量的减少可以降低手术过程中的应激反应,从而降低术后并发症的发生,因此减少术中出血量可以有效地促进患者术后恢复[15]。此外,腹腔镜手术可以显著增加淋巴结清扫数量(20.3 vs. 17.2枚, P=0.030),这可能是由于腹腔镜手术下视野清晰、解剖精细,可以获得更多的淋巴结清扫数量。在术后恢复方面,先前的研究证实了腹腔镜手术具有创伤小、术后恢复快的特点[16-18]。Vignali等研究结果表明腹腔镜手术可以显著缩短首次排气时间、首次进流食时间与术后住院时间[18]。与上述报道一致,本研究发现,与开腹手术相比,高龄结直肠癌患者接受腹腔镜手术可以明显缩短排气时间、经口进食时间与术后住院时间。腹腔镜较小的手术创伤可以减少患者术后疼痛,便于患者更早下床活动,在加快患者排气时间与进食时间的同时,可以减少高龄患者术后血管栓塞风险,提高围手术期安全性。综上所述,腹腔镜手术可以为高龄结直肠癌患者提供更加精细的手术操作,降低出血量、减少术后并发症、提高淋巴结检出数量、加快术后恢复,其短期治疗优势明显。
目前,鲜有文献报道高龄结直肠癌患者行腹腔镜手术的远期预后[19-20]。2015年,Hinoi等研究发现80岁以上高龄结直肠癌患者行腹腔镜与开腹手术的3年OS、DFS与肿瘤特异性生存率相似[20]。与之相似的是,2016年Moon等也报道了腹腔镜手术可以提供与开腹结直肠癌手术相似的远期治疗效果[19]。本研究发现行开腹手术与腹腔镜手术的高龄结直肠癌患者的5年OS(66.2% vs. 71.4%, P=0.594)与DFS(60.4% vs. 72.8%, P=0.295)差异无统计学意义。然而需要注意的是,腹腔镜组患者的5年OS与DFS有普遍高于开腹组患者的趋势,尤其是腹腔镜组患者的5年DFS要高出开腹组患者12%。这种生存差异可能是由于腹腔镜组与开腹组患者的淋巴结清扫数量不同导致的,淋巴结清扫数量是评价不同术式之间肿瘤学治疗效果与预后的客观指标。因此,虽然在统计学方面无差异,但高龄结直肠癌患者行腹腔镜可能有着更好的肿瘤学治疗效果,在充分清扫肿瘤周围淋巴组织的同时,可以准确评估病理分期,能潜在提高高龄结直肠癌患者的生存预后。
影响结直肠癌患者生存的预后因素文献已有报道[21-24]。Huh等研究报道,术前CEA水平、TNM分期、血管侵犯和神经侵犯是潜在影响结直肠癌患者根治性术后OS和DFS的独立不良预后因素[24]。此外,Tasi等研究结果显示神经侵犯是Ⅱ期结直肠癌患者根治性手术不良预后因素[21]。与既往文献报道相似,本研究对匹配后的患者预后进行分析,结果发现CEA水平、TNMⅢ期、神经侵犯是影响高龄结直肠癌患者根治性术后不良OS与DFS的独立危险因素。
综上所述,与开腹手术相比,腹腔镜手术可以为80岁以上高龄结直肠癌患者提供更为明显的短期优势和相似的远期预后。在无明显腹腔镜禁忌证的前提下,推荐高龄结直肠癌患者优先选择腹腔镜手术。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:毛争强、杜波涛:方案实施、数据收集及论文撰写孙航、周力:数据收集与统计学分析郭得兴:论文指导与修改李新宇、宰守峰:方案设计、论文审校与修改 -
表 1 匹配前、后开腹组与腹腔镜组老年患者临床病理特征(n(%))
Table 1 Clinicopathological features of elderly patients in the open surgery and laparoscopic surgery groups before and after matching (n(%))

表 2 匹配后开腹组与腹腔镜组老年患者围手术期结局
Table 2 Perioperative outcomes of elderly patients in the open surgery and laparoscopic surgery groups after matching

表 3 匹配后开腹组与腹腔镜组老年患者病理结果(n(%))
Table 3 Pathologic outcomes of elderly patients in the open surgery and laparoscopic surgery groups after matching (n(%))

表 4 匹配后老年患者总体生存率与无瘤生存率的单因素与多因素Cox回归分析
Table 4 Univariate and multivariate Cox regression analyses of overall survival and disease-free survival in elderly patients after matching

-
[1] Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors[J]. Prz Gastroenterol, 2019, 14(2): 89-103.
[2] Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. doi: 10.3322/caac.21492
[3] Athanasiou CD, Robinson J, Yiasemidou M, et al. Laparoscopic vs Open approach for transverse colon cancer. A systematic review and meta-analysis of short and long term outcomes[J]. Int J Surg, 2017, 41: 78-85. doi: 10.1016/j.ijsu.2017.03.050
[4] Parker JM, Feldmann TF, Cologne KG. Advances in Laparoscopic Colorectal Surgery[J]. Surg Clin North Am, 2017, 97(3): 547-560. doi: 10.1016/j.suc.2017.01.005
[5] Durak D, Alkurt EG, Turhan VB, et al. Comparison of Short-Term Results of Laparoscopic and Open Surgeries for Colorectal Cancer: A Single-Center Experience[J]. Cureus, 2022, 14(5): e24635.
[6] Hinoi T, Kawaguchi Y, Hattori M, et al. Laparoscopic versus open surgery for colorectal cancer in elderly patients: a multicenter matched case-control study[J]. Ann Surg Oncol, 2015, 22(6): 2040-2050. doi: 10.1245/s10434-014-4172-x
[7] Zhou S, Wang X, Zhao C, et al. Laparoscopic vs. open colorectal cancer surgery in elderly patients: short- and long-term outcomes and predictors for overall and disease-free survival[J]. BMC Surg, 2019, 19(1): 137. doi: 10.1186/s12893-019-0596-3
[8] Fujii S, Ishibe A, Ota M, et al. Long-term results of a randomized study comparing open surgery and laparoscopic surgery in elderly colorectal cancer patients (Eld Lap study)[J]. Surg Endosc, 2021, 35(10): 5686-5697. doi: 10.1007/s00464-020-08026-0
[9] Numata M, Sawazaki S, Morita J, et al. Comparison of Laparoscopic and Open Surgery for Colorectal Cancer in Patients with Severe Comorbidities[J]. Anticancer Res, 2018, 38(2): 963-967.
[10] Luo W, Wu M, Chen Y. Laparoscopic versus open surgery for elderly patients with colorectal cancer: a systematic review and meta-analysis of matched studies[J]. ANZ J Surg, 2022, 92(9): 2003-2017. doi: 10.1111/ans.17972
[11] Chen B, Yu W, Ma Y, et al. Evaluation of the safety and efficacy of perform enterectomy in colorectal cancer patients aged 80 or older. A meta-analysis and a systematic review[J]. Int J Colorectal Dis, 2023, 38(1): 185. doi: 10.1007/s00384-023-04461-2
[12] Son IT, Kim JY, Kim MJ, et al. Clinical and oncologic outcomes of laparoscopic versus open surgery in elderly patients with colorectal cancer: a retrospective multicenter study[J]. Int J Clin Oncol, 2021, 26(12): 2237-2245. doi: 10.1007/s10147-021-02009-4
[13] Ogata T, Yoshida N, Sadakari Y, et al. Colorectal cancer surgery in elderly patients 80 years and older: a comparison with younger age groups[J]. J Gastrointest Oncol, 2022, 13(1): 137-148. doi: 10.21037/jgo-21-627
[14] Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience[J]. Ann Surg, 2009, 250(2): 187-196. doi: 10.1097/SLA.0b013e3181b13ca2
[15] Zhang X, Wu Q, Gu C, et al. Comparison of short and long-time outcomes between laparoscopic and conventional open multivisceral resection for primary T4b colorectal cancer[J]. Asian J Surg, 2019, 42(1): 401-408. doi: 10.1016/j.asjsur.2018.06.010
[16] Feng B, Zheng MH, Mao ZH, et al. Clinical advantages of laparoscopic colorectal cancer surgery in the elderly[J]. Aging Clin Exp Res, 2006, 18(3): 191-195. doi: 10.1007/BF03324648
[17] She WH, Poon JT, Fan JK, et al. Outcome of laparoscopic colectomy for cancer in elderly patients[J]. Surg Endosc, 2013, 27(1): 308-312. doi: 10.1007/s00464-012-2466-2
[18] Vignali A, Di Palo S, Tamburini A, et al. Laparoscopic vs. open colectomies in octogenarians: a case-matched control study[J]. Dis Colon Rectum, 2005, 48(11): 2070-2075. doi: 10.1007/s10350-005-0147-0
[19] Moon SY, Kim S, Lee SY, et al. Laparoscopic surgery for patients with colorectal cancer produces better short-term outcomes with similar survival outcomes in elderly patients compared to open surgery[J]. Cancer Med, 2016, 5(6): 1047-1054. doi: 10.1002/cam4.671
[20] Hinoi T, Kawaguchi Y, Hattori M, et al. Laparoscopic versus open surgery for colorectal cancer in elderly patients: a multicenter matched case-control study[J]. Ann Surg Oncol, 2015, 22(6): 2040-2050. doi: 10.1245/s10434-014-4172-x
[21] Tsai HL, Cheng KI, Lu CY, et al. Prognostic significance of depth of invasion, vascular invasion and numbers of lymph node retrievals in combination for patients with stage Ⅱ colorectal cancer undergoing radical resection[J]. J Surg Oncol, 2008, 97(5): 383-387. doi: 10.1002/jso.20942
[22] Mehrkhani F, Nasiri S, Donboli K, et al. Prognostic factors in survival of colorectal cancer patients after surgery[J]. Colorectal Dis, 2009, 11(2): 157-161. doi: 10.1111/j.1463-1318.2008.01556.x
[23] Fujita S, Shimoda T, Yoshimura K, et al. Prospective evaluation of prognostic factors in patients with colorectal cancer undergoing curative resection[J]. J Surg Oncol, 2003, 84(3): 127-131. doi: 10.1002/jso.10308
[24] Huh JW, Oh BR, Kim HR, et al. Preoperative carcinoembryonic antigen level as an independent prognostic factor in potentially curative colon cancer[J]. J Surg Oncol, 2010, 101(5): 396-400. doi: 10.1002/jso.21495



 下载:
下载: