-
摘要:目的
了解胃癌危险因素研究中孟德尔随机化(MR)方法的应用情况,为胃癌的病因学研究和预防策略制定提供依据。
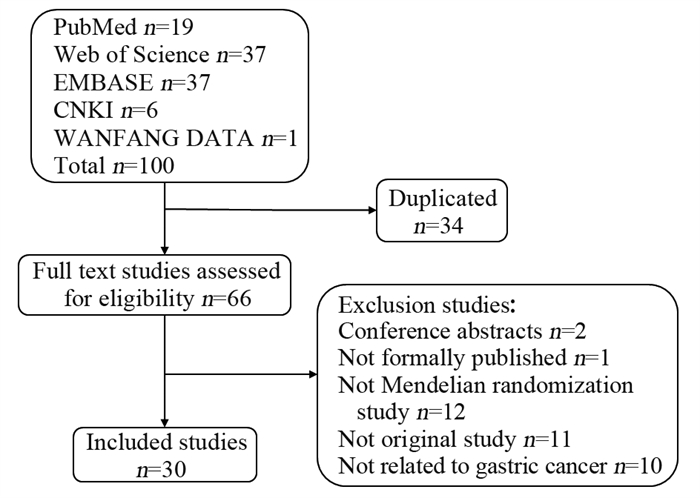
方法在PubMed、Web of Science、EMBASE、中国知网和万方数据检索建库至2022年11月19日收录的基于MR方法的胃癌危险因素研究。由两位作者独立筛选文献、摘录信息并进行质量评价。
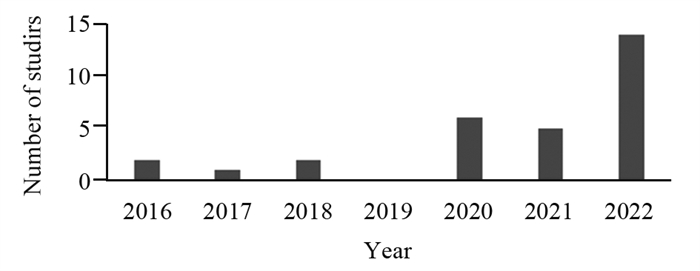
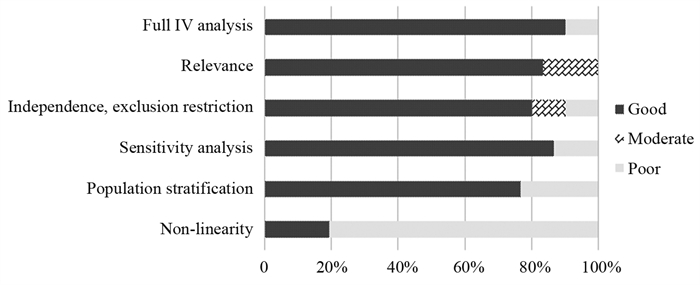
结果共纳入2016—2022年发表的30篇研究报告,其中20篇被判定为高质量研究。这些研究探讨了行为及生活方式、人体测量特征、机体生物暴露指标及其他疾病罹患情况与胃癌的关系,支持吸烟等因素可能与胃癌发生风险存在因果关联。
结论既往MR研究广泛探讨了机体内外暴露因素或表型与胃癌发生的因果关联,为胃癌病因学研究提供了积极证据。但MR研究可能受方法学缺陷制约,需结合其他证据,对结果谨慎解读。
Abstract:ObjectiveTo understand the application and research progress of Mendelian randomization (MR) studies related to gastric cancer and provide a scientific basis for gastric cancer prevention.
MethodsPublished studies on risk factors of gastric cancer based on MR methods were searched in PubMed, Web of Science, EMBASE, CNKI, and WANFANG DATA from the establishment of each database to November 19th, 2022. Two researchers examined the eligibility of studies, extracted key information, and assessed the research quality independently.
ResultsA total of 30 publications published from 2016 to 2022 were included in the study, and 20 were judged to be of high quality. These studies examined the relationship between behaviors and lifestyle factors, anthropometric characteristics, indicators of biological exposure, and other pathological conditions and gastric cancer, and the results suggest potential causal associations between smoking and other factors and the risk of gastric cancer.
ConclusionPrevious MR studies extensively investigated the causal association between internal and external exposures or traits and gastric cancer and provided positive evidence of gastric cancer etiology. However, MR studies may be subject to methodological limitations. Interpretation of results needs to be approached with caution, which necessitates the integration with biological plausibility and evidence from observation studies.
-
Key words:
- Gastric cancer /
- Mendelian randomization /
- Causality
-
0 引言
热休克转录因子1(heat shock transcription factor 1, HSF1)是调控热休克蛋白(heat-shock proteins, HSPs)表达的转录因子。细胞未受刺激时,HSF1以单体形式在细胞核和细胞质之间穿梭,当细胞受到刺激时,游离的HSF1以三聚体的形式磷酸化,并入核诱导靶基因的转录[1],在热激过程中Ser326位点的HSF1磷酸化可以正向调控转录。在此期间,HSF1与错误折叠的蛋白质被蛋白酶体降解,并延迟热休克反应的衰减直到清除受损的蛋白质为止[2]。
FBXW7(F-box and WD repeat domain containing 7)是F-box家族的重要成员之一,为SCF(SKP1-Cullin1-F-box)E3泛素连接酶复合物的靶蛋白识别组分[3]。FBXW7是继K-RAS和APC之后研究发现在结直肠癌中最常见的突变基因之一[4]。研究发现结直肠癌组织中FBXW7的表达水平低于正常组织,其失活会诱导癌症的发生[5]。约7.5%的转移性结直肠腺癌患者中发现FBXW7错义突变,与结直肠癌不良预后有关[6]。FBXW7介导多种癌蛋白的降解,如NF-κB、c-Myc、Cyclin E、Notch1、c-Jun和Brg1等[7]。FBXW7位于人4号染色体长臂(4q31.3)上,分为FBXW7α、FBXW7β和FBXW7γ三个亚型,其中FBXW7α位于细胞核中,FBXW7β位于细胞质中,而FBXW7γ位于核仁中[8]。FBXW7α通过与GSK3β激活的Ser303和Ser307位点磷酸化HSF1结合,介导HSF1泛素化降解。有研究发现缺失FBXW7α导致黑色素瘤细胞核HSF1蛋白表达水平明显升高,而对细胞质HSF1表达没有影响[9]。然而,FBXW7对结直肠癌细胞胞质和胞核内的HSF1在热刺激后及未刺激状态下的表达定位的调控尚不清楚。本研究利用细胞免疫荧光法观察敲除FBXW7对温热刺激及恢复期间HSF1在胞质和胞核中的定位的影响,明确FBXW7在HSF1及激活的HSF1在胞核中的表达定位的调控作用。
1 材料与方法
1.1 细胞株及试剂
人结直肠癌细胞系HCT116、DLD1及HCT116 WT、HCT116 FBXW7 KO、DLD1 WT及DLD1 FBXW7 KO、真核表达质粒PcDNA3-HA-FBXW7α[10]均由美国哈佛医学院贝斯以色列女执事医疗中心病理系魏文毅教授馈赠。FBXW7基因敲除的HCT116和DLD1细胞由Bert Vogelstein和Christoph Lengauer构建,得到Bert Vogelstein的馈赠[11]。主要通过利用细菌人工染色体(BAC)系统进行基因克隆,并通过Cre/loxP敲除系统和单克隆细胞筛选获得FBXW7基因敲除的HCT116和DLD1细胞[12]。结直肠癌细胞均用含10%胎牛血清的DMEM培养液置于37℃、5%CO2的培养箱中培养。胎牛血清、DMEM高糖培养基、Opti-MEM减血清培养基购自美国Gibco公司,BCA试剂盒(货号P0011)及蛋白质提取相关试剂均购自上海碧云天公司,转染试剂Lipofectamine3000(货号L3000075)购自美国Invitrogen公司。
1.2 细胞转染
取对数生长期的结直肠癌细胞株DLD1接种于6个60 mm的培养皿中,接种密度以过夜培养后细胞融合度为70%~90%为准。按照Lipofectamine3000说明书提供的操作流程,以Opti-MEM减血清培养基配制转染试剂及质粒工作液,将PcDNA3-HA-FBXW7α真核表达质粒转染入DLD1细胞中。转染6 h后更换含10%胎牛血清的完全培养基继续培养。
1.3 Western blot法检测过表达FBXW7α基因对HSF1蛋白表达水平的影响
收集转染24 h后的DLD1细胞,加入细胞裂解液RIPA提取细胞总蛋白,用BCA法检测蛋白质浓度,通过10%SDS-PAGE分离蛋白,采用90V电压将分离蛋白条带转移到活化的PVDF膜。用含5%脱脂牛奶的TBST溶液封闭膜1.5 h,然后加入兔抗人HSF1(CST,货号4356,1:1000)、兔抗人pHSF1Ser326单克隆抗体(Invitrogen,货号MA5-32105,1:1000)及鼠抗人β-actin(CST,货号3700,1:1000)、HA(Sigma,货号H9658,1:1000)单克隆抗体4℃孵育过夜;加入HRP标记的羊抗鼠IgG(CST,货号7076,1:5000)及羊抗兔IgG(CST,货号7074,1:5000)。滴加化学发光HRP底物ECL发光液(Millipore,货号WBKLS0500),使用MiniChemi 610Plus型化学发光成像系统进行曝光显影。实验重复3次。
1.4 Western blot法检测FBXW7对结直肠癌细胞中HSF1蛋白表达的影响
收集HCT116WT、HCT116 FBXW7 KO、DLD1 WT及DLD1 FBXW7 KO细胞, 加入细胞裂解液RIPA提取细胞总蛋白,取对数生长期细胞进行42℃水浴温热刺激1 h,并在37℃培养恢复30 min和60 min,用核蛋白提取试剂盒提取细胞核蛋白和胞质蛋白。用BCA法检测蛋白质浓度,通过10%SDS-PAGE分离蛋白,采用90V电压将分离蛋白条带转移到活化的PVDF膜。用含5%脱脂牛奶的TBST溶液封闭膜1.5 h,然后加入兔抗人HSF1(CST,货号4356,1:1000)、兔抗人pHSF1Ser326单克隆抗体(Invitrogen,货号MA5-32105,1:1000)及鼠抗人β-actin(CST,货号3700,1:1000)、Lamin A/C(CST,货号4777,1:1000)单克隆抗体4℃孵育过夜;加入HRP标记的羊抗鼠IgG(CST,货号7076,1:5000)及羊抗兔IgG(CST,货号7074,1:5000),滴加化学发光HRP底物ECL发光液(Millipore,货号WBKLS0500),使用MiniChemi 610Plus型化学发光成像系统进行曝光显影。实验重复3次。
1.5 细胞免疫荧光法检测FBXW7对激活的HSF1在结直肠癌细胞中表达定位的影响
取对数生长期的HCT116 WT、HCT116 FBXW7 KO、DLD1 WT及DLD1 FBXW7 KO细胞,制备细胞爬片。待细胞融合度达90%时,进行42℃水浴温热刺激1 h,并在37℃培养恢复30 min和60 min,用4%多聚甲醛溶液室温固定20 min;用0.3%Triton X-100室温通透20 min;3%BSA室温封闭1 h;加入兔抗人HSF1(CST,货号4356,1:200)和pHSF1Ser326单克隆抗体(Invitrogen,货号MA5-32105,1:200)4℃过夜;加入Alexa Fluor 488标记(Invitrogen,货号A-11008,1:400)及Alexa Fluor 555标记(Invitrogen,货号A-21428,1:400)的羊抗兔IgG,避光反应2 h;加入DAPI荧光封片剂对细胞核进行染色及封片,荧光显微镜(日本奥林巴斯BX53)观察HSF1的表达位置变化。实验重复3次。
1.6 统计学方法
应用SPSS23.0统计学软件对实验结果进行统计学分析。所有实验独立重复3次,结果数据以(x±s)表示。两组间比较采用独立样本t检验,以P < 0.05为差异有统计学意义。
2 结果
2.1 FBXW7α基因过表达结直肠癌细胞中pHSF1Ser326蛋白的表达水平
与对照组相比,DLD1细胞转染PcDNA3-HA-FBXW7α真核表达质粒后,pHSF1Ser326蛋白的表达水平显著降低(P=0.0003),见图 1。提示FBXW7α可以靶向下调HSF1的蛋白表达水平。
2.2 缺失FBXW7对HSF1和pHSF1Ser326蛋白在胞质和胞核表达的影响
Western blot检测结果显示,DLD1 FBXW7 KO细胞的HSF1总蛋白的表达水平高于DLD1 WT细胞(P=0.029),HCT116 FBXW7 KO细胞的HSF1总蛋白的表达水平高于HCT116 WT细胞(P=0.039);HCT116 FBXW7 KO细胞中pHSF1Ser326总蛋白的表达水平显著高于HCT116 WT细胞(P=0.019),见图 2A。进一步采用细胞免疫荧光法观察到FBXW7 KO细胞中HSF1和pHSF1Ser326主要聚集在胞核,在胞质中仅有少量表达。结果表明,FBXW7的缺失导致HSF1和pHSF1Ser326总蛋白表达水平显著升高,并且主要在胞核中表达,见图 2B~C。
![]() 图 2 FBXW7的缺失导致结直肠癌细胞中HSF1和pHSF1Ser326主要在胞核中累积Figure 2 Loss of FBXW7 resulted in accumulation of HSF1 and pHSF1Ser326 mainly in nucleus of CRC cellsA: the expression levels of HSF1 protein and pHSF1Ser326 protein in CRC cells were detected by Western blot; B: immunofluorescent staining of HSF1 protein and β-actin protein in CRC cells; C: immunofluorescent staining of pHSF1Ser326 protein in CRC cells (B, C×400, staining with 4, 6-diamidino-2-phenylindole (DAPI), Alexa Fluor 488 and Alexa Fluor 555, scale bar=50μm); n=3; WT: wild type.
图 2 FBXW7的缺失导致结直肠癌细胞中HSF1和pHSF1Ser326主要在胞核中累积Figure 2 Loss of FBXW7 resulted in accumulation of HSF1 and pHSF1Ser326 mainly in nucleus of CRC cellsA: the expression levels of HSF1 protein and pHSF1Ser326 protein in CRC cells were detected by Western blot; B: immunofluorescent staining of HSF1 protein and β-actin protein in CRC cells; C: immunofluorescent staining of pHSF1Ser326 protein in CRC cells (B, C×400, staining with 4, 6-diamidino-2-phenylindole (DAPI), Alexa Fluor 488 and Alexa Fluor 555, scale bar=50μm); n=3; WT: wild type.2.3 缺失FBXW7对温热刺激后HSF1在胞核中累积时间的影响
对结直肠癌细胞42℃温热刺激持续1 h,并在37℃培养指定时间。细胞免疫荧光法检测结果显示,温热刺激细胞后HSF1在细胞核中表达迅速增强,恢复30 min后,HCT116 WT和DLD1 WT细胞中的核HSF1表达逐渐恢复至未刺激水平,FBXW7 KO的两种细胞中HSF1在核中表达较强,恢复60 min后仍然可以看到在核中表达较强,见图 3。表明FBXW7的缺失导致温热刺激细胞后HSF1恢复至未刺激水平的时间延长。
![]() 图 3 FBXW7的缺失使温热刺激细胞后HSF1在胞核中累积时间延长Figure 3 Loss of FBXW7 prolonged accumulation of HSF1 in nucleus after warm stimulationA, B: the expression of HSF1 in the nucleus and cytoplasm of CRC cells during recovery from heat shock were detected by immunofluorescence staining method (×400, staining with 4', 6-diamidino-2-phenylindole (DAPI) and Alexa Fluor 488, scale bar=50μm).
图 3 FBXW7的缺失使温热刺激细胞后HSF1在胞核中累积时间延长Figure 3 Loss of FBXW7 prolonged accumulation of HSF1 in nucleus after warm stimulationA, B: the expression of HSF1 in the nucleus and cytoplasm of CRC cells during recovery from heat shock were detected by immunofluorescence staining method (×400, staining with 4', 6-diamidino-2-phenylindole (DAPI) and Alexa Fluor 488, scale bar=50μm).2.4 FBXW7调控激活的HSF1在胞质和胞核中的表达定位
对结直肠癌细胞分别进行42℃温热刺激持续1 h,并在37℃恢复指定时间。Western blot结果显示,温热刺激后37℃恢复培养60 min组(Recovery 60 min组)和37℃培养对照组(Untreated组)的DLD1 WT细胞中的pHSF1Ser326蛋白表达水平差异无统计学意义(P=0.305),即恢复60 min后DLD1 WT细胞逐渐恢复至未刺激水平。与Untreated组相比,Recovery 60 min组的DLD1细胞的FBXW7 KO细胞核中pHSF1Ser326蛋白表达水平明显上调(P=0.007),HCT116细胞的FBXW7 KO细胞核中pHSF1Ser326蛋白表达水平也显著上调(P=0.002),见图 4A~B。细胞免疫荧光结果显示,温热刺激后FBXW7 KO细胞中的pHSF1Ser326在细胞核中表达均高于WT细胞,37℃培养60 min后,HCT116 WT和DLD1 WT细胞恢复至未刺激水平,FBXW7 KO细胞中的pHSF1Ser326在胞核中表达仍然很强,在胞质中表达较弱,见图 4C~D。以上结果表明,热激后HSF1表达水平恢复受阻可能与缺失FBXW7不能降解核HSF1有关,FBXW7可能调控激活及衰减期间的HSF1在胞质和胞核中的表达定位。
![]() 图 4 FBXW7调控激活的HSF1在胞质和胞核中的表达定位Figure 4 FBXW7 regulated the expression and the localization of activate HSF1 in cytoplasm and nucleus.A, B: the expression levels of pHSF1Ser326 protein in the nucleus were detected by Western blot; C, D: the expression of pHSF1Ser326 was detected using fluorescence microscopy(×400, staining with 4', 6-diamidino-2-phenylindole (DAPI) and Alexa Fluor 488, scale bar=50μm); **: P < 0.01, ***: P < 0.001, n=3; 1: Untreated; 2: Heat shock 42℃ 1h; 3: Recovery 30min; 4: Recovery 60min
图 4 FBXW7调控激活的HSF1在胞质和胞核中的表达定位Figure 4 FBXW7 regulated the expression and the localization of activate HSF1 in cytoplasm and nucleus.A, B: the expression levels of pHSF1Ser326 protein in the nucleus were detected by Western blot; C, D: the expression of pHSF1Ser326 was detected using fluorescence microscopy(×400, staining with 4', 6-diamidino-2-phenylindole (DAPI) and Alexa Fluor 488, scale bar=50μm); **: P < 0.01, ***: P < 0.001, n=3; 1: Untreated; 2: Heat shock 42℃ 1h; 3: Recovery 30min; 4: Recovery 60min3 讨论
热休克反应(heat shock response, HSR)是一种细胞保护机制,可防止由于温热刺激、缺氧、细胞内pH值波动或暴露于重金属等的刺激引起的蛋白质错误折叠。HSR由转录因子HSF1诱导热休克蛋白的动态转录过程。在未受刺激的细胞中,HSF1处于失活状态;当受到应激刺激时,HSF1在细胞核中被磷酸化,形成同源三聚体,结合DNA并激活热休克基因转录[1]。HSF1的激活和恢复期间受到多种翻译后修饰的调节,尤其是磷酸化对HSF1调控转录具有重要作用。Ser121、Ser303、Ser307以及Ser363磷酸化负调控HSF1的转录活性,而mTOR、MEK和P38 MAPK可以催化Ser326的磷酸化从而激活HSF1[13]。基质中磷酸化HSF1的激活与卵巢癌、慢性淋巴细胞性白血病和骨髓瘤患者的预后不良有关[14]。另有研究发现,细胞核中HSF1的表达水平增高与结直肠癌、乳腺癌、肾癌和口腔鳞状细胞癌的转移和不良的生存率有关[15]。因此,深入研究激活后的HSF1在细胞核中的表达水平及定位对肿瘤的治疗具有重要意义。
既往研究表明,FBXW7对HSF1的泛素化和降解与肿瘤细胞耐药有关[16]。在胃癌中,细胞核中的代谢酶PDK3以破坏HSF1磷酸化的方式阻止FBXW7介导的HSF1蛋白酶体降解,PDK3与HSF1相互作用形成正反馈回路以促进糖酵解[17]。近期有研究发现,ERK1/2在耐药细胞中可降低FBXW7的表达,并且通过抑制HSF1的泛素化降解导致MDR1的转录激活[18]。FBXW7参与HSF1靶向肠缺血再灌注期间的泛素化和降解,表明FBXW7可能是抑制肠缺血再灌注期间肠黏膜凋亡的潜在治疗靶标[19]。Nikos等[9]发现HSF1可以与FBXW7α结合,是FBXW7α的泛素化底物,通过Western blot实验发现缺失FBXW7的结直肠癌细胞HCT116在基础状态下,核HSF1蛋白的表达水平增高,胞质HSF1蛋白表达无变化;而温热刺激后的HSF1蛋白在核中表达水平显著增高,且热休克反应时间延长;他们还发现缺失FBXW7导致黑色素瘤细胞WM39的核HSF1表达水平明显升高,而对细胞质HSF1表达无影响,温热刺激后核HSF1蛋白表达水平增强,恢复期间缺失FBXW7的WM39细胞中的HSF1在核中累积[9]。HSF1在胞核中的累积是由于HSF1在胞质和胞核中的动态转移变化,还是温热刺激时FBXW7不调控HSF1的降解,导致HSF1在核中累积,还尚不清楚。
本研究采用细胞免疫荧光法对敲除FBXW7的结直肠癌细胞进行42℃温热刺激并观察HSF1的表达定位,此细胞免疫荧光HSF1的定位结果与文献[9]所报道的缺失FBXW7导致HSF1蛋白在核中累积的Western blot结果一致。为进一步探讨FBXW7是否调节激活的HSF1在胞核中的表达和定位,通过细胞免疫荧光实验发现,敲除FBXW7的细胞温热刺激并恢复37℃培养30 min后,pHSF1Ser326在胞核中的表达仍较强,表明缺失FBXW7导致细胞核中的HSF1降解受阻。有研究表明,HSF1基础表达主要在细胞核内[1]。研究发现,Western blot实验表明HSF1在胞质有较强的表达,而免疫荧光实验却显示HSF1主要定位在细胞核。这可能是由于分离细胞核和细胞质所用的裂解液造成的,大多数以单体形式存在的HSF1可能在裂解作用下从细胞核中提取出来,而三聚体的HSF1在提取过程中仍与细胞核DNA紧密结合[20]。
综上所述,本研究证实了FBXW7可调控HSF1及激活的磷酸化HSF1在胞质和胞核中的表达和定位。推测FBXW7可能成为结直肠癌治疗的潜在分子调控靶点。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:王梦圆:研究设计与实施、论文构思与撰写许恒敏:研究设计指导、文章审阅及修改汪靖暄:文献检索与筛选、信息提取与核查潘凯枫:文章审阅及修改李文庆:研究设计指导、文章审阅及修改 -
表 1 英文数据库文献检索策略汇总
Table 1 Summary of literature search strategies in English databases

表 2 孟德尔随机化研究质量评价方法
Table 2 Methods for assessing the quality of MR study

表 3 20篇高质量胃癌危险因素孟德尔随机化研究的主要信息
Table 3 Key information of 20 high-quality MR studies related to risk factors of gastric cancer

-
[1] Li WQ, Zhang JY, Ma JL, et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial[J]. BMJ, 2019, 366: l5016. http://www.bmj.com/content/366/bmj.l5016
[2] Thrift AP, El-Serag HB. Burden of Gastric Cancer[J]. Clin Gastroenterol Hepatol, 2020, 18(3): 534-542. doi: 10.1016/j.cgh.2019.07.045
[3] Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease?[J]. Int J Epidemiol, 2003, 32(1): 1-22. doi: 10.1093/ije/dyg070
[4] 毛盈颖, 俞飞, 汪天培, 等. 体脂含量与胃癌发生的孟德尔随机化研究[J]. 浙江中医药大学学报, 2018, 42(5): 333-338. doi: 10.16466/j.issn1005-5509.2018.05.001 Mao YY, Yu F, Wang TP, et al. Body Fat Percentage and Risk of Gastric Cancer-a Mendelian Randomization Study[J]. Zhejiang Zhong Yi Yao Da Xue Xue Bao, 2018, 42(5): 333-338. doi: 10.16466/j.issn1005-5509.2018.05.001
[5] Larsson SC, Carter P, Kar S, et al. Smoking, alcohol consumption, and cancer: A mendelian randomisation study in UK Biobank and international genetic consortia participants[J]. PLoS Med, 2020, 17(7): e1003178. doi: 10.1371/journal.pmed.1003178
[6] Yuan S, Mason AM, Titova OE, et al. Morning chronotype and digestive tract cancers: Mendelian randomization study[J]. Int J Cancer, 2022, 152(4): 697-704. http://pubmed.ncbi.nlm.nih.gov/35061662/
[7] Titova OE, Michaelsson K, Vithayathil M, et al. Sleep duration and risk of overall and 22 site-specific cancers: A Mendelian randomization study[J]. Int J Cancer, 2021, 148(4): 914-920. doi: 10.1002/ijc.33286
[8] Carter P, Yuan S, Kar S, et al. Coffee consumption and cancer risk: a Mendelian randomisation study[J]. Clin Nutr, 2022, 41(10): 2113-2123. doi: 10.1016/j.clnu.2022.08.019
[9] Wu K, Liu L, Shu T, et al. The relationship between processed meat, red meat, and risk of types of cancer: A Mendelian randomization study[J]. Front Nutr, 2022, 9: 942155. doi: 10.3389/fnut.2022.942155
[10] Vithayathil M, Carter P, Kar S, et al. Body size and composition and risk of site-specific cancers in the UK Biobank and large international consortia: A mendelian randomisation study[J]. PLoS Med, 2021, 18(7): e1003706. doi: 10.1371/journal.pmed.1003706
[11] Mao YY, Yan CW, Lu Q, et al. Genetically predicted high body mass index is associated with increased gastric cancer risk[J]. Eur J Hum Genet, 2017, 25(9): 1061-1066. doi: 10.1038/ejhg.2017.103
[12] Zang ZP, Shao Y, Nakyeyune R, et al. Association of Body Mass Index and the Risk of Gastro-Esophageal Cancer: A Mendelian Randomization Study in a Japanese Population[J]. Nutr Cancer, 2022: 1-10.
[13] Wade KH, Carslake D, Sattar N, et al. BMI and Mortality in UK Biobank: Revised Estimates Using Mendelian Randomization[J]. Obesity(Silver Spring), 2018, 26(11): 1796-1806. http://www.onacademic.com/detail/journal_1000040894364610_c353.html
[14] Jiang H, Hu DJ, Wang J, et al. Adiponectin and the risk of gastrointestinal cancers in East Asians: Mendelian randomization analysis[J]. Cancer Med, 2022, 11(12): 2397-2404. doi: 10.1002/cam4.4735
[15] Song J, Li A, Qian Y, et al. Genetically Predicted Circulating Levels of Cytokines and the Risk of Cancer[J]. Front Immunol, 2022, 13: 886144. doi: 10.3389/fimmu.2022.886144
[16] Gao Y, Wei Y, Zhou X, et al. Assessing the Relationship Between Leukocyte Telomere Length and Cancer Risk/Mortality in UK Biobank and TCGA Datasets With the Genetic Risk Score and Mendelian Randomization Approaches[J]. Front Genet, 2020, 11: 583106. doi: 10.3389/fgene.2020.583106
[17] Yuan S, Kar S, Carter P, et al. Is Type 2 Diabetes Causally Associated With Cancer Risk? Evidence From a Two-Sample Mendelian Randomization Study[J]. Diabetes, 2020, 69(7): 1588-1596. doi: 10.2337/db20-0084
[18] Zhang H, Li D, Liu X, et al. Fasting Insulin and Risk of Overall and 14 Site-Specific Cancers: Evidence From Genetic Data[J]. Front Oncol, 2022, 12: 863340. doi: 10.3389/fonc.2022.863340
[19] Zhu M, Ma Z, Zhang X, et al. C-reactive protein and cancer risk: a pan-cancer study of prospective cohort and Mendelian randomization analysis[J]. BMC Med, 2022, 20(1): 301. doi: 10.1186/s12916-022-02506-x
[20] Wu YL, Xin JY, Loehrer EA, et al. High-density lipoprotein, low-density lipoprotein and triglyceride levels and upper gastrointestinal cancers risk: a trans-ancestry Mendelian randomization study[J]. Eur J Clin Nutr, 2022, 76(7): 995-1002. doi: 10.1038/s41430-022-01078-6
[21] Yuan S, Carter P, Vithayathil M, et al. Genetically predicted circulating B vitamins in relation to digestive system cancers[J]. Br J Cancer, 2021, 124(12): 1997-2003. doi: 10.1038/s41416-021-01383-0
[22] Larsson SC, Mason AM, Vithayathil M, et al. Circulating vitamin C and digestive system cancers: Mendelian randomization study[J]. Clin Nutr, 2022, 41(9): 2031-2035. doi: 10.1016/j.clnu.2022.07.040
[23] Gu D, Zhang M, Wang Y, et al. Causal effect of autoimmune liver diseases on cancer: meta-analyses of cohort studies and Mendelian randomization study[J]. Liver Int, 2022, 42(10): 2216-2226. doi: 10.1111/liv.15355
[24] Kamiza AB, Fatumo S, Singini MG, et al. Hepatitis B infection is causally associated with extrahepatic cancers: A Mendelian randomization study[J]. EBioMedicine, 2022, 79: 104003. doi: 10.1016/j.ebiom.2022.104003
[25] Fadista J, Yakimov V, Võsa U, et al. Genetic regulation of spermine oxidase activity and cancer risk: a Mendelian randomization study[J]. Sci Rep, 2021, 11(1): 17463. doi: 10.1038/s41598-021-97069-x
[26] Larsson SC, Carter P, Vithayathil M, et al. Insulin-like growth factor-1 and site-specific cancers: A Mendelian randomization study[J]. Cancer Med, 2020, 9(18): 6836-6842. doi: 10.1002/cam4.3345
[27] Larsson SC, Carter P, Vithayathil M, et al. Genetically predicted plasma phospholipid arachidonic acid concentrations and 10 site-specific cancers in UK biobank and genetic consortia participants: A mendelian randomization study[J]. Clin Nutr, 2021, 40(5): 3332-3337. doi: 10.1016/j.clnu.2020.11.004
[28] Larsson SC, Kar S, Perry JRB, et al. Serum Estradiol and 20 Site-Specific Cancers in Women: Mendelian Randomization Study[J]. J Clin Endocrinol Metab, 2022, 107(2): e467-e474. doi: 10.1210/clinem/dgab713
[29] Niu WQ, Pang Q, Lin T, et al. A Causal Role of Genetically Elevated Circulating Interleukin-10 in the Development of Digestive Cancers: Evidence from Mendelian Randomization Analysis Based on 29, 307 Subjects[J]. Medicine(Baltimore), 2016, 95(7): e2799. http://pubmed.ncbi.nlm.nih.gov/26886630/
[30] Wang TP, Ren CL, Ni J, et al. Genetic Association of Plasma Homocysteine Levels with Gastric Cancer Risk: A Two-Sample Mendelian Randomization Study[J]. Cancer Epidemiol Biomarkers Prev, 2020, 29(2): 487-492. doi: 10.1158/1055-9965.EPI-19-0724
[31] Xu W, Cheng YL, Zhu HR. Evaluation of an Association of Blood Homocysteine Levels With Gastric Cancer Risk From 27 Case-Control Studies[J]. Medicine(Baltimore), 2016, 95(20): e3700. http://www.scienceopen.com/document_file/e6cda1e6-d54e-4163-8ff4-6cb5e4e74faf/PubMedCentral/e6cda1e6-d54e-4163-8ff4-6cb5e4e74faf.pdf
[32] Yin L, Yan H, Chen K, et al. Diet-Derived Circulating Antioxidants and Risk of Digestive System Tumors: A Mendelian Randomization Study[J]. Nutrients, 2022, 14(16): 3274. doi: 10.3390/nu14163274
[33] Yuan S, Vithayathil M, Kar S, et al. Assessing the protective role of allergic disease in gastrointestinal tract cancers using Mendelian randomization analysis[J]. Allergy, 2021, 76(5): 1559-1562. doi: 10.1111/all.14616
[34] Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement[J]. JAMA, 2021, 326(16): 1614-1621. doi: 10.1001/jama.2021.18236
[35] van de Luitgaarden IAT, van Oort S, Bouman EJ, et al. Alcohol consumption in relation to cardiovascular diseases and mortality: a systematic review of Mendelian randomization studies[J]. Eur J Epidemiol, 2022, 37(7): 655-669. doi: 10.1007/s10654-021-00799-5
[36] Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration[J]. BMJ, 2021, 375: n2233. http://www.xueshufan.com/publication/3210406414
[37] 高雪, 薛付忠, 黄丽红, 等. 孟德尔随机化模型及其规范化应用的统计学共识[J]. 中国卫生统计, 2021, 38(3): 471-475, 480. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGWT202103042.htm Gao X, Xue F, Huang L, et al. Statistical consensus on mendelian randomization model and its standardized application[J]. Zhongguo Wei Sheng Tong Ji, 2021, 38(3): 471-475, 480. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGWT202103042.htm
[38] Smith GD. Reflections on the limitations to epidemiology[J]. J Clin Epidemiol, 2001, 54(4): 325-331. doi: 10.1016/S0895-4356(00)00334-6
[39] Nelson CP, Hamby SE, Saleheen D, et al. Genetically determined height and coronary artery disease[J]. N Engl J Med, 2015, 372(17): 1608-1618. doi: 10.1056/NEJMoa1404881
[40] Guo Y, Li ZX, Zhang JY, et al. Association Between Lifestyle Factors, Vitamin and Garlic Supplementation, and Gastric Cancer Outcomes: A Secondary Analysis of a Randomized Clinical Trial[J]. JAMA Netw Open, 2020, 3(6): e206628. doi: 10.1001/jamanetworkopen.2020.6628
[41] Ladeiras-Lopes R, Pereira AK, Nogueira A, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies[J]. Cancer Causes Control, 2008, 19(7): 689-701. doi: 10.1007/s10552-008-9132-y
[42] Turati F, Tramacere I, La Vecchia C, et al. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma[J]. Ann Oncol, 2013, 24(3): 609-617. doi: 10.1093/annonc/mds244
[43] Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies[J]. Hum Mol Genet, 2014, 23(R1): R89-R98. doi: 10.1093/hmg/ddu328
-
期刊类型引用(1)
1. 刘艳春,贾儒渊,樊志慧,王朝凤,苏海燕,曹文娟,徐丹丹. 表皮生长因子受体在食管鳞状细胞癌组织中的表达及其临床意义. 临床误诊误治. 2024(10): 35-38+43 .  百度学术
百度学术
其他类型引用(0)




 下载:
下载: