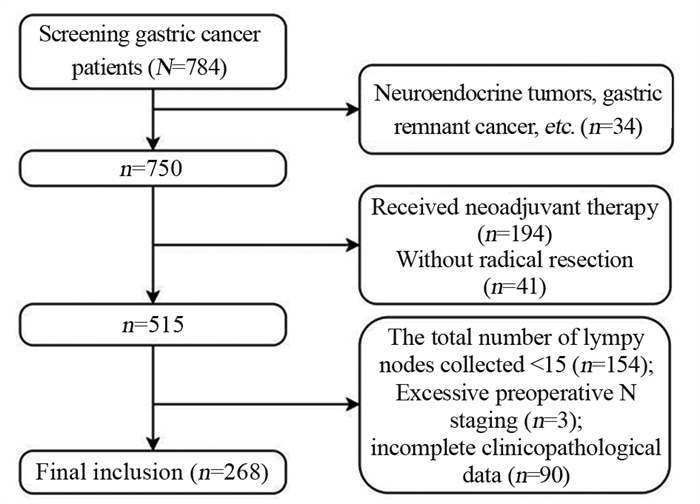
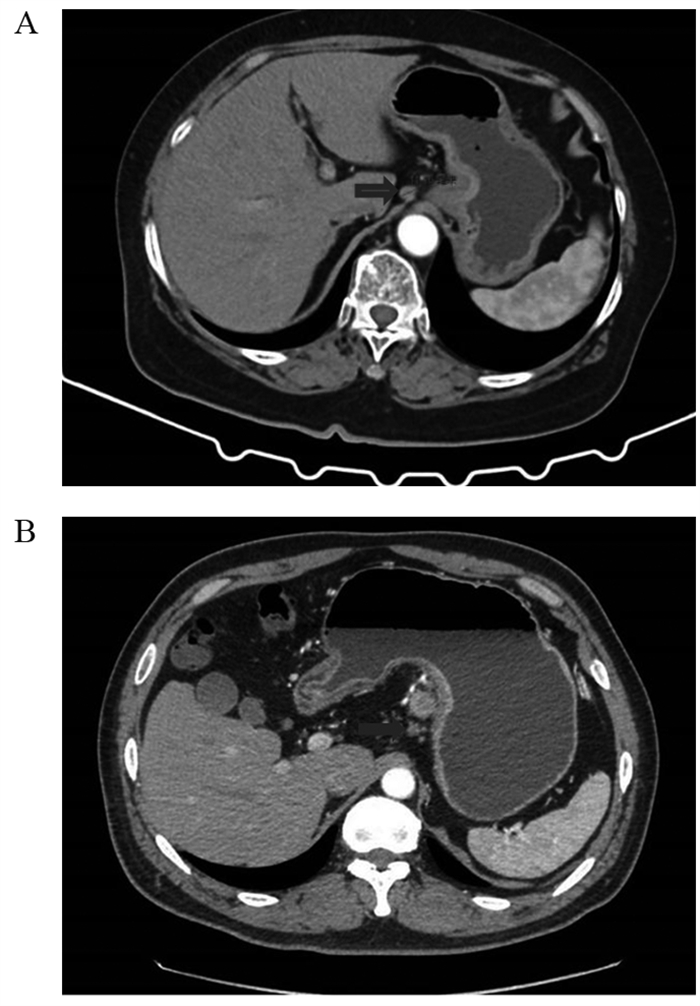
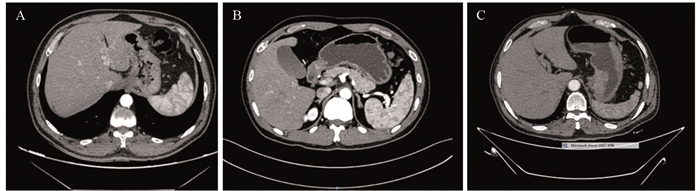
Risk Factors and Establishment of Prediction Model for Preoperative Lymph Node Staging Deficiency in Gastric Cancer
-
摘要:目的
分析胃癌术前淋巴结分期(N分期)不足的危险因素,建立术前评估模型,辅助预测术前N分期。
方法回顾性分析268例胃癌患者的临床病理资料。患者术前常规行薄层增强CT评估术前N分期,结合术后病理结果分析术前N分期不足的危险因素。Logistic回归分析筛选出的影响因素,Kaplan-Meier绘制术前N分期准确组与不足组的生存曲线,利用R软件包绘制Nomogram图、预测模型的ROC曲线,计算AUC、95%CI、敏感度和特异性。
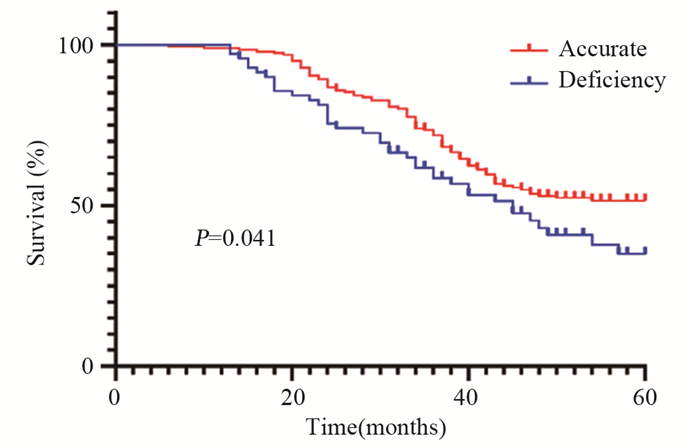
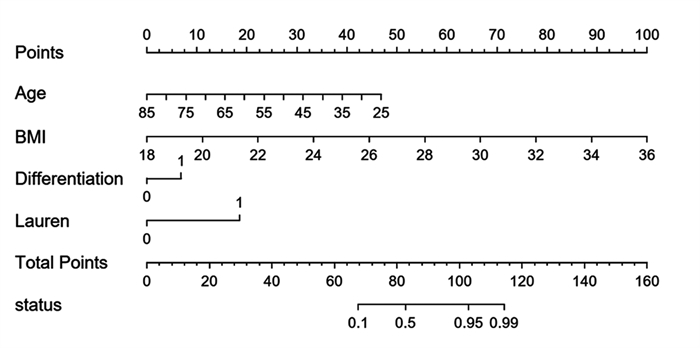
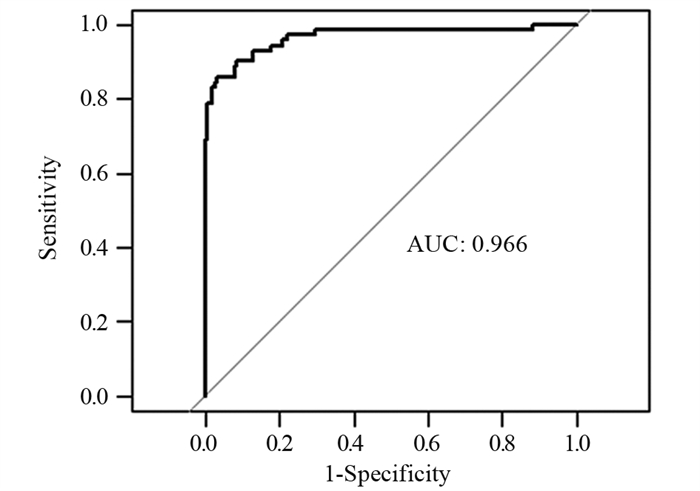
结果年龄、BMI、低分化、Lauren分型为弥漫型是胃癌术前N分期不足的独立危险因素(P < 0.05)。术前N分期不足组预后生存显著差于准确组(P=0.041)。预测模型的ROC曲线AUC为0.935,敏感度为85.9%,特异性为96.9%。
结论年龄越小、BMI越高、低分化、Lauren分型为弥漫型是术前N分期不足的独立危险因素。本研究基于年龄、BMI、分化程度、Lauren分型建立的术前N分期评估模型,具有较高的可信度。
Abstract:ObjectiveTo analyze the risk factors of preoperative lymph node staging (N-stage) deficiency in gastric cancer and establish a preoperative assessment model to assist in predicting preoperative N-stage.
MethodsA retrospective method was used to analyze the clinicopathological data of 268 patients with gastric cancer. The patients routinely underwent preoperative thin-section enhanced CT to assess preoperative N-stage. Results The risk factors for preoperative N-stage deficiency were analyzed in combination with postoperative pathological findings. Multifactorial logistic regression analysis was performed to determine influencing factors, and Kaplan-Meier analysis was used to plot the survival curves of preoperative N-stage accurate group and deficiency group. The nomogram plot and ROC curves of the prediction model were drawn using the R package. AUC, 95%CI, sensitivity, and specificity were calculated.
ResultsAge, BMI, poor differentiation, and Lauren's classification as diffuse were independent risk factors for preoperative N-stage deficiency in gastric cancer (P < 0.05). Prognostic survival was significantly worse in the preoperative N stage-inadequate group than that in the accurate group (P=0.041). The AUC area was 0.935, with a sensitivity of 85.9% and specificity of 96.9%.
ConclusionYoung age, high BMI, poor differentiation, and Lauren's classification as diffuse are independent risk factors for preoperative N-stage deficiency. The established preoperative assessment model based on age, BMI, differentiation degree, and Lauren's classification in this study has relatively high credibility.
-
0 引言
子宫内膜癌是在北美和欧洲最常见的女性恶性肿瘤,近年来我国的发病率明显增加,发病人群年轻化[1]。子宫内膜癌的发病机制目前尚未完全明确,随着研究的深入,越来越清晰地认识到子宫内膜癌是多种因素协同交叉作用、具有不同遗传和分子特征的一类疾病[2-3]。根据肿瘤发病机制与雌激素依赖相关性,将其分为Ⅰ型和Ⅱ型,80%以上为Ⅰ型子宫内膜癌。遗传易感性是导致个体对相同致癌因素敏感度不一致的重要因素,某些雌激素代谢通路中关键酶可以改变体内雌激素或外源性雌激素及其代谢产物的水平。基因多态性与酶活性有关,雌激素代谢酶基因多态性可能导致子宫内膜癌发病易感性差异[4]。本研究利用SNP分型检测技术,探索雌激素代谢关键酶CYP1B1、CYP1A1和NQO1基因的单核苷酸多态性(single nucleotide polymorphisms, SNPs)位点分布频率与Ⅰ型子宫内膜癌易感性之间关系,以期对Ⅰ型子宫内膜癌的易感人群进行筛查。
1 资料与方法
1.1 临床资料
抽取我院2014年3月—2016年10月收治的经病理诊断为子宫内膜样腺癌的103例患者和同期子宫内膜正常的100例其他疾病患者静脉外周全血2~3 ml,作为检测标本。病例和对照组均取得患者的知情同意并详细询问病史,包括初潮年龄、生育状况、绝经年龄、孕产次数、内科并发症及肿瘤家族史。测量身高与体重,并计算体质指数(body mass index, BMI),测量血压,采集静脉血化验空腹血糖(FPG)及血脂、肿瘤标志物[5-6]。年龄31~78岁,平均(51.15±9.69)岁,子宫内膜癌组平均年龄(54.77±8.21)岁,对照组为(46.95±9.65)岁。
1.2 方法
1.2.1 确定候选基因及其SNPs位点
由于Ⅰ型子宫内膜癌为雌激素依赖性肿瘤,结合相关文献报道,在美国国家生物技术信息中心(National Center for Biotechnology Information, NCBI)人类基因组数据库中选择与雌激素代谢相关的关键酶CYP1A1、CYP1B1,具有抗癌突变的依赖还原型辅酶Ⅰ/Ⅱ醌氧化还原酶1(quinone oxido-reductase1, NQO1)3个基因,选取与代谢酶活性密切相关且杂合度大于10%的SNPs位点进行基因型分析。
1.2.2 引物设计合成
利用引物设计软件premier 5.0,在含SNPs的DNA序列上设计PCR引物,并将设计的引物进行引物的同源性比较,选出同源性最小而Tm值和G/C比值合适的引物作为PCR反应引物,所扩增片段应包含要检测的SNPs位点,其位置尽可能设计在PCR扩增片段的中部。
1.2.3 检测方法
使用DNA提取试剂盒(DP304-03)从外周血中提取DNA,进行预扩增,琼脂糖凝胶电泳检测DNA完整性。根据设计引物模板,进行延伸反应及延伸产物纯化。PCR扩增反应体系总体积为50 μl,内含5 μl 10×PCR缓冲液,3 μl 25 mmol/L MgCl2溶液,5 μl 2 mmol/L dNTP混合物,1.25 U Taq DNA聚合酶,0.5 μmol/L引物及100g的基因组DNA。反应在MJ公司PTC-100 PCR反应仪中进行。PCR循环条件为95℃预变性10 min,然后94℃变性30 s,60℃复性30 s,72℃延伸30 s,反应40个循环后,72℃再延伸10 min。扩增产物用2%的琼脂糖凝胶电泳检测,4℃保存。PCR产物经纯化后作为测序模板,用Big-Dye末端荧光标记试剂盒进行测序反应,测序反应产物经纯化后在ABI公司3730XL测序仪上进行测序电泳,用Gene codes公司的Sequencer4.2对测序结果进行分析。
1.3 统计学方法
采用SPSS18.0软件对实验数据进行统计学分析,用χ2检验和多因素Logistic回归模型分析各基因型在两组人群中的分布差异及其与子宫内膜癌临床病理特征的相关性,P < 0.05为差异有统计学意义。
2 结果
2.1 多因素回归分析
CYP1B1基因SNPs位点rs111888224因无碱基改变未纳入统计,rs1056836、rs2551188、rs10916在研究人群中均存在多态性,但分布频率差异无统计学意义;rs1056836 C、G基因分布频率差异有统计学意义(P=0.0454)。
CYP1A1基因SNP位点rs4646421在研究人群中存在CT、CC、TT多态性。与CT型相比,CC型为保护基因型,OR=0.479(95%CI: 0.255~0.899),差异有统计学意义(P=0.0219),其C、T基因分布频率差异有统计学意义(P=0.0041),见表 1。
表 1 基因型/等位基因在Ⅰ型子宫内膜癌患者中的分布(n(%))Table 1 Genotype/alleles distributions in typeⅠendometrial carcinoma cases (n(%))
2.2 CYP1A1基因rs4646421位点多态性与Ⅰ型子宫内膜癌危险因素的分层分析
对CYP1A1基因rs4646421位点不同基因型与Ⅰ型子宫内膜癌的危险因素相关性进行分层比较,发现在年龄 > 60岁、BMI≥25、绝经延迟、并发高血压的个体中,携带CT+TT突变基因型将增加罹患Ⅰ型子宫内膜癌的风险(P < 0.05),见表 2。
表 2 发病危险因素与基因型分布的关系(n(%))Table 2 Association between risk factors and genotypes distributions of SNP(rs4646421) in CYP1A1 (n(%))
3 讨论
雌激素分布在细胞内外表面,通过雌激素受体的核内结合蛋白在靶细胞中保持高亲和力和特异性。涉及雌激素生物合成和代谢的基因的多态性是雌激素受体阳性恶性肿瘤的潜在危险因素,有几种细胞色素P450(CYP)酶参与雌激素的氧化代谢途径[7]。CYP1A1和CYP1B1是代谢产生2-羟基-4-羟基雌激素代谢物的基本酶[8-9],在不同人群中不仅存在多态性,并且在性激素相关组织中的表达高于细胞色素P450超家族的其他成员,其基因多态性与乳腺癌、子宫肌瘤、子宫内膜异位症、宫颈癌等疾病风险相关[10-14]。
CYP1A1基因定位于人类染色体15q22-24,编码由512个氨基酸组成的蛋白质,是细胞色素P450家族中高诱导成员。CYP1A1酶的激活参与内源性和外源性化合物的氧化作用,催化多环芳香族碳氢化合物转化为酚与环氧化合物。一些酚类和环氧化合物可与DNA结合形成加合物,最终转化为致癌物、二醇环氧化物,通过环氧化物水解酶与一些抗肿瘤药物发生耐药,并增加个体肿瘤风险[9, 15]。本研究结果显示CYP1A1基因SNP位点rs4646421在子宫内膜癌人群中存在CT、CC、TT多态性,与携带CT基因型相比,携带CC基因型的个体罹患子宫内膜癌的风险较小,携带T等位基因的个体子宫内膜癌的发病风险高于C等位基因携带者。与携带CC基因型相比,携带TC+TT基因型在年龄 > 60岁、BMI≥25、绝经延迟(超过52岁)、合并高血压的女性中Ⅰ型子宫内膜癌的发病风险增加。
CYP1B1基因位于染色体2q21-22,其单核苷酸多态性SNP总数有353个,目前研究较多的是SNPrs1056836,其mRNA发生1294位碱基胞嘧啶(cytosine, C)变异为鸟嘌呤(guanine, G),使DNA两条链形成野生CC型,杂合CG型和变异GG型3种基因型,即基因多态性。这种改变导致第三外显子432密码子的碱基由CTG变异为GTG,由其编码的亮氨酸(leucine, Leu)变异为缬氨酸(valine, Val),导致CYP1B1酶蛋白功能的改变[16]。CYP1B1通过多方面影响肿瘤的易感性和预后,在胰岛素抵抗中产生作用,突变型表达的酶之蛋白质表现出更强的激活癌前物质活性,或影响睾酮的6β-羟化作用及性激素的激活代谢等[17-18]。
本研究中CYP1B1基因SNP rs2551188、rs10916两个位点在人群中存在多态性,但分布频率无统计学差异;rs1056836在正常子宫内膜组与子宫内膜癌两组间的基因型分布频率无差异,但发生更多的碱基胞嘧啶变异为鸟嘌呤,可能会使罹患子宫内膜癌的风险增加,以往文献中发现对于单个位点基因突变的表现为阴性结论时,多个位点联合的单倍体基因型研究却往往能得到阳性结论,似乎预示着CYP1B1基因在分子遗传学上有一定的倾向性[16-19],但其与肿瘤易感性及预后的机制问题还存在着诸多争议与疑惑,有待于进一步研究。
NQO1是一种重要的化学致癌物质代谢酶,具有抗癌作用,可使内源性及外源性醌类化合物通过电子还原反应变成低毒性的氢醌类物质,再转化为水溶性化合物排出体外,降低了细胞发生突变及癌变的概率。如果位点发生突变,使原位编码的氨基酸发生改变,将会导致该酶活性减弱或完全丧失,降低解毒功能且不能维护细胞稳定,增加某些易感个体细胞发生癌变[20]。报道显示NQO1蛋白高表达在卵巢癌、乳腺癌、结直肠癌等肿瘤中,与临床分期晚、分化程度差和淋巴结转移等临床特征有关[21-22],本研究选择的SNP位点rs45488899和rs1800566虽在两组间均存在基因多态性,但分布频率不具有差异性,因此认为这两个SNP位点与Ⅰ型子宫内膜癌的发生无显著相关性。
本研究通过基因表达谱差异的分析,推测CYP1A1基因rs4646421位点的多态性在Ⅰ型子宫内膜癌的发生发展中起着重要作用,可作为进行高危人群筛查的基因位点,还可以突变的基因型为基础,采取针对性的基因靶向治疗,或者通过修正基因突变谱来改善其预后。但是,基因多态性受复杂的遗传、环境因素、种族等的影响,并与样本量的大小有关,必须经过大样本试验来进一步明确基因多态性与子宫内膜癌易感性之间的基因环境交互作用及具体机制。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:杜书祥:撰写文章郭振、陈紫来、王尚鑫:数据搜索和分析吴 刚:研究指导、论文修改 -
表 1 患者临床病理资料(n(%))
Table 1 Clinicopathological data of patients (n(%))

表 2 胃癌术前N分期不足多因素分析
Table 2 Multivariate analysis of preoperative N stage deficiency in gastric cancer

-
[1] Sung H, Ferllay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries[J]. CA J Clin, 2021, 71(3): 209-249. doi: 10.3322/caac.21660
[2] 陈博晋, 胡星怡, 赵菁文, 等. 免疫治疗在胃癌新辅助治疗中的研究进展[J]. 肿瘤防治研究, 2022, 49(7): 727-732. doi: 10.3971/j.issn.1000-8578.2022.21.1241 Cheng BJ, Hu XY, Zhao JW, et al. Current Status of Immunotherapy in Neoadjuvant Therapy for Gastric Cancer[J]. Zhong Liu Fang Zhi Yan Jiu, 2022, 49(7): 727-732. doi: 10.3971/j.issn.1000-8578.2022.21.1241
[3] Su PF, Yu JC. Progress in neoadjuvant therapy for gastric cancer[J]. Oncol Lett, 2022, 23(6): 172. doi: 10.3892/ol.2022.13292
[4] Panduro-Correa V, Cubas WS, Herrera-Matta JJ, et al. Survival and adequate preoperative staging in patients undergoing gastric cancer surgery at a Peruvian Police Hospital[J]. J Surg Oncol, 2021, 123(2): 425-431. doi: 10.1002/jso.26315
[5] 钟宝元, 刘艳秀, 黄文峰, 等. 64层CT三期动态增强扫描对胃癌术前TNM分期的判断价值[J]. 中华胃肠外科杂志, 2012, 15(7): 706-709. https://www.cnki.com.cn/Article/CJFDTOTAL-YXYY201903073.htm Zhong BY, Liu YX, Huang WF, et al. Clinical value of 64-slice spiral 3-phase CT enhanced scanning for preoperative TNM staging assessment of gastric carcinoma[J]. Zhonghua Wei Chang Wai Ke Za Zhi, 2012, 15(7): 706-709. https://www.cnki.com.cn/Article/CJFDTOTAL-YXYY201903073.htm
[6] In H, Solsky I, Palis B, et al. Validation of the 8th Edition of the AJCC TNM Staging System for Gastric Cancer using the National Cancer Database[J]. Ann Surg Oncol, 2017, 24(12): 3683-3691. doi: 10.1245/s10434-017-6078-x
[7] Chen YC, Fang WL, Wang RF, et al. Clinicopathological Variation of Lauren Classification in Gastric Cancer[J]. Pathol Oncol Res, 2016, 22(1): 197-202. doi: 10.1007/s12253-015-9996-6
[8] Chen Z, Liu Y, Dou L, et al. The efficacy of the application of the curative criteria of the 5(rd) edition Japanese gastric cancer treatment guidelines for early adenocarcinoma of the esophagogastric junction treated by endoscopic submucosal dissection[J]. Saudi J Gastroenterol, 2021, 27(2): 97-104. doi: 10.4103/sjg.SJG_403_20
[9] Jiang ZY, Kinami S, Nakamura N, et al. Diagnostic ability of multi-detector spiral computed tomography for pathological lymph node metastasis of advanced gastric cancer[J]. World J Gastrointest Oncol, 2020, 12(4): 435-446. doi: 10.4251/wjgo.v12.i4.435
[10] Lim JS, Yun MJ, Kim MJ, et al. CT and PET in stomach cancer: preoperative staging and monitoring of response to therapy[J]. Radiographics, 2006, 26(1): 143-156. doi: 10.1148/rg.261055078
[11] Wang FH, Zhang XT, Li YF, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021[J]. Cancer Commun (Lond), 2021, 41(8): 747-795. doi: 10.1002/cac2.12193
[12] 刘颖斌, 董平, 吴文广. 加强胃癌的分期研究[J]. 中华普通外科杂志, 2013, 28(10): 729-731. Liu YB, Dong P, Wu WG. Strengthening the staging study of gastric cancer[J]. Zhonghua Pu Tong Wai Ke Za Zhi, 2013, 28(10): 729-731.
[13] Láinez Ramos-Bossini AJ, Ruiz Carazo E, Rabadán Caravaca MD. 'Back-and-Forth Stomach' CT Imaging Findings of a Pathophysiologic Entity Causing Acute Gastric Volvulus[J]. Tomography, 2022, 8(1): 245-256. doi: 10.3390/tomography8010019
[14] 武乐斌, 王锡明, 李振家, 等. 螺旋CT成像技术在胃癌CT分期及术前评估中的价值[J]. 中国医学影像技术, 2001, 17(4): 301-303. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXX200104004.htm Wu LB, Wang XM, Li ZJ, et al. Evaluation of Spiral CT Imaging Technique in Staging of Gastric Carcinoma and Preoperative Assessment[J]. Zhongguo Yi Xue Ying Xiang Ji Shu, 2001, 17(4): 301-303. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXX200104004.htm
[15] 郗新娟, 曹波. 多层螺旋CT在胃癌术前分期诊断中的应用[J]. 延安大学学报(医学科学版), 2021, 19(1): 76-79. https://www.cnki.com.cn/Article/CJFDTOTAL-YAXY202101019.htm Xi XJ, Cao B. Application of multislice spiral CT in the diagnosis of preoperative staging of gastric cancer[J]. Yan'An Da Xue Xue Bao (Yi Xue Ke Xue Ban), 2021, 19(1): 76-79. https://www.cnki.com.cn/Article/CJFDTOTAL-YAXY202101019.htm
[16] Huang CM, Xu M, Wang JB, et al. Is tumor size a predictor of preoperative N staging in T2-T4a stage advanced gastric cancer?[J]. Surg Oncol, 2014, 23(1): 5-10. doi: 10.1016/j.suronc.2014.01.003
[17] Ajani JA, Bentrem DeJ, Besh S, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines[J]. J Natl Compr Canc Netw, 2013, 11(5): 531-546. doi: 10.6004/jnccn.2013.0070
[18] Yamashita K, Hosoda K, Ema A, et al. Lymph node ratio as a novel and simple prognostic factor in advanced gastric cancer[J]. Eur J Sur Oncol, 2016, 42(9): 1253-1260. doi: 10.1016/j.ejso.2016.03.001
[19] 夏晨梅, 陈霞, 李倩倩, 等. 超声内镜对胃癌术前T、N分期准确率的评估及其影响因素分析[J]. 浙江医学, 2018, 40(3): 255-257, 265. https://www.cnki.com.cn/Article/CJFDTOTAL-ZJYE201803010.htm Xia CM, Chen X, Li QQ, et al. Accuracy of endoscopic ultrasonography in preoperative staging for patients with gastric cancer and its influencing factors[J]. Zhejiang Yi Xue, 2018, 40(3): 255-257, 265. https://www.cnki.com.cn/Article/CJFDTOTAL-ZJYE201803010.htm
[20] Ahmad A, Khan H, Cholankeril G, et al. The impact of age on nodal metastases and survival in gastric cancer[J]. J Surg Res, 2016, 202(2): 428-435. doi: 10.1016/j.jss.2016.02.043
[21] Malaguarnera L, Cristaldi E, Malaguarnera M. The role of immunity in elderly cancer[J]. Crit Rev Oncol Hematol, 2010, 74(1): 40-60. http://www.cabdirect.org/abstracts/20123220853.html;jsessionid=86F0FA4D2715A555A0542CBDC2A8A227;jsessionid=CCBFF5A5FB054BD18720053DDA12A472
[22] Nienhueser H, Kunzmann R, Sisic L, et al. Surgery of gastric cancer and esophageal cancer: Does age matter?[J]. J Surg Oncol, 2015, 112(4): 387-395. http://www.onacademic.com/detail/journal_1000039192532810_32a8.html
[23] Misleh JG, Santoro P, Strasser JF, et al. Multidisciplinary management of gastric cancer[J]. Surg Oncol Clin N Am, 2013, 22(2): 247-264. http://www.onacademic.com/detail/journal_1000036210827210_2663.html
[24] Comstock SS, Hortos K, Kovan B, et al. Adipokines and obesity are associated with colorectal polyps in adult males: a cross-sectional study[J]. PLoS One, 2014, 9(1): e85939. http://www.science-open.com/document_file/84f37265-e29b-4357-9cba-8518ba66bcc8/PubMedCentral/84f37265-e29b-4357-9cba-8518ba66bcc8.pdf
[25] Sikalidis AK, Varamini B. Roles of hormones and signaling molecules in describing the relationship between obesity and colon cancer[J]. Pathol Oncol Res, 2011, 17(4): 785-790. http://www.onacademic.com/detail/journal_1000034551387910_3e79.html
[26] Kwee RM, Kwee TC. Predicting lymph node status in early gastric cancer[J]. Gastric Cancer, 2008, 11(3): 134-148. doi: 10.1007%2Fs10120-008-0476-5.pdf
[27] Watanabe M, Kato J, Inoue I, et al. Development of gastric cancer in nonatrophic stomach with highly active inflammation identified by serum levels of pepsinogen and Helicobacter pylori antibody together with endoscopic rugal hyperplastic gastritis[J]. Int J Cancer, 2012, 131(11): 2632-2642. http://europepmc.org/abstract/MED/22383377
[28] Kluijt I, Siemerink EJ, Ausems MG, et al. CDH1-related hereditary diffuse gastric cancer syndrome: clinical variations and implications for counseling[J]. Int J Cancer, 2012, 131(2): 367-376. http://www.xueshufan.com/publication/2035731110
[29] Donner I, Kiviluoto T, Ristimäki A, et al. Exome sequencing reveals three novel candidate predisposition genes for diffuse gastric cancer[J]. Fam Cancer, 2015, 14(2): 241-246. http://www.onacademic.com/detail/journal_1000037349256410_5b49.html
[30] Adachi Y, Yasuda K, Inomatam, et al. Pathology and prognosis of gastric carcinoma: well versus poorly differentiated type[J]. Cancer, 2000, 89(7): 1418-1424. http://bu.edu.eg/portal/uploads/discussed_thesis/11203725/11203725_R.pdf
[31] Wang Y, Liu W, Yu Y, et al. CT radiomics nomogram for the preoperative prediction of lymph node metastasis in gastric cancer[J]. Eur Radiol, 2020, 30(2): 976-986.
[32] Feng QX, Liu C, Qi L, et al. An Intelligent Clinical Decision Support System for Preoperative Prediction of Lymph Node Metastasis in Gastric Cancer[J]. J Am Coll Radiol, 2019, 16(7): 952-960.
[33] Chen S, Nie RC, Ouyang LY, et al. Nomogram analysis and external validation to predict the risk of lymph node metastasis in gastric cancer[J]. Oncotarget, 2017, 8(7): 11380-11388.
[34] Saito T, Kurokawa Y, Takiguchi S, et al. Accuracy of multidetector-row CT in diagnosing lymph node metastasis in patients with gastric cancer[J]. Eur Radiol, 2015, 25(2): 368-374.
[35] 中国肿瘤临床学会指南工作委员会. 2022年CSCO胃癌诊疗指南[M]. 北京: 人民卫生出版社, 2022. Guidelines Working Committee of Chinese Clinical Society of Oncology. Guidelines for the diagnosis and treatment of gastric cancer in CSCO in 2022[M]. Beijing: People's Medical Publishing House, 2022.



 下载:
下载: