Role of Preoperative Peripheral Blood Inflammatory Parameters and Postoperative Lymph-node Ratio in Prognosis of Patients with Gastric Cancer Undergoing Chemotherapy
-
摘要:目的
分析术前外周血炎性参数与术后淋巴结比率(LNR)在胃癌化疗患者预后中的作用。
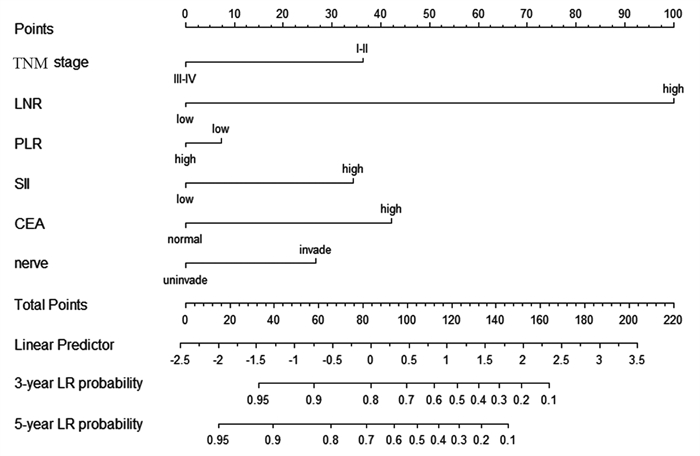
方法按外周血中性粒细胞与淋巴细胞比率(NLR)、血小板-淋巴细胞比率(PLR)、全身免疫炎性反应指数(SII)、淋巴细胞-单核细胞比率(LMR)、小野寺预后营养指数(OPNI)、LNR截断值将108例胃癌患者分为高低两组,分析这些炎性参数对胃癌化疗患者总生存期(OS)的预后价值。以独立预后指标绘制列线图预测胃癌患者的存活率。
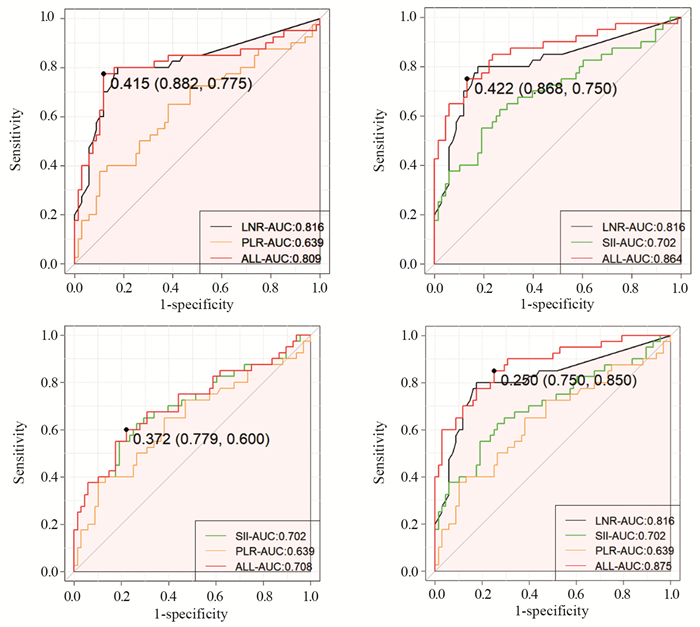
结果NLR在胃癌化疗患者的预后评估中有统计学意义(P < 0.001),高PLR组、高SII组、高LNR组、N3分期、TNM(Ⅲ~Ⅳ)分期、神经侵犯、癌胚抗原(CEA)是胃癌预后的独立危险因素(均P < 0.05)。LNR、PLR及SII三者组合(AUC=0.875)在胃癌预后方面的预测效果优于单独或两两联合。
结论NLR、LNR、PLR及SII升高与患者的生存期显著减少相关;LNR、PLR及SII可作为胃癌化疗患者预后的独立危险因素,三者联合可预测胃癌患者的生存期。
Abstract:ObjectiveTo analyze the role of preoperative peripheral blood inflammatory parameters and postoperative lymph-node ratio (LNR) in the prognosis of gastric cancer patients treated with chemotherapy.
MethodsThe neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), systemic immune inflammatory response index (SII), lymphocyte-to-monocyte ratio (LMR), Onodera prognostic nutritional index (OPNI), and LNR of 108 patients with gastric cancer were classified into high and low groups to analyze their prognostic value on the overall survival (OS) of gastric cancer patients treated with chemotherapy. The independent prognostic indicators were plotted in columns to predict the survival rate of gastric cancer patients.
ResultsNLR was statistically significant in the prognostic assessment of gastric cancer patients treated with chemotherapy (P < 0.001). Moreover, the high PLR group, high SII group, high LNR group, N3 stage, TNM (Ⅲ-Ⅳ) stage, nerve invasion, and carcinoembryonic antigen (CEA) were independent risk factors for the prognosis of gastric cancer (all P < 0.05). These findings indicated that in predicting the prognosis of gastric cancer, the combination of LNR, PLR, and SII (AUC=0.875) was better than that of LNR, PLR, and SII alone or in pairwise combination.
ConclusionElevated NLR, LNR, PLR, and SII are associated with significantly reduced survival of patients. LNR, PLR, and SII could be used as independent risk factors for the prognosis of gastric cancer patients treated with chemotherapy, and their combination can predict the survival of gastric cancer patients.
-
Key words:
- LNR /
- Peripheral blood inflammatory parameters /
- Gastric cancer /
- Prognosis
-
0 引言
环状RNA(circular RNA, circRNA)是一类共价封闭的内源性非编码RNA,无5′端帽子和3′端多聚A尾,具有组织特异性,广泛存在于人类细胞中[1]。circRNA在肿瘤等多种疾病相关组织及细胞中存在表达差异,提示circRNA对肿瘤相关疾病具有调节作用[2]。世界卫生组织最新数据显示,原发性肝癌发病率居恶性肿瘤第6位,死亡率居第3位。肝细胞癌(hepatocellular carcinoma, HCC)是原发性肝癌的主要类型,约占肝癌总数的85%~90%[3-6]。目前,HCC的早期临床筛查方法主要是甲胎蛋白(AFP)测定和肝脏超声检查,但AFP检测的敏感度和特异性有限,而超声检查很大程度上依赖操作者的主观判断,且常规超声难以准确鉴别肝脏占位性病变性质[7]。BCLC分期系统显示,仅有少数患者在肝癌早期阶段,可接受如局部消融、切除或肝移植等治疗,预后较好;大多数患者就诊时多属于晚期,尚无有效治疗方案选择[8]。因此,亟需深入探究HCC的发生发展机制,寻找新型生物标志物以提高HCC的早期检出率,这对HCC患者的早期诊治、改善预后、提高生存率及生存质量具有重要意义。近年来随着高通量测序技术和生物信息学分析技术的快速发展,发现许多与HCC相关的circRNA,该技术逐渐应用于临床诊断和基础研究中,进一步提示circRNA将成为HCC新型诊断标志物和药物开发靶点[9]。本文就近年来circRNA的生物学机制及在HCC中发挥的作用、诊治研究进展等进行综述。
1 circRNA的概述
1.1 circRNA的分类和特性
circRNAs是生物体内存在的一类不具有5'末端帽子和3'末端poly(A)尾、能通过共价键形成闭合环形结构的RNA分子。它广泛存在于多种生物体中,在细胞中已发现超过10万个circRNA。它具有结构稳定、种类丰富、进化保守等特性,表达水平具有组织、时序特异性等。circRNA的来源主要有三种形式:(1)套索驱动环化,由外显子的3'剪接供体与5'剪接受体共价形成套索,套索进行内部拼接后剪除内含子形成circRNA(exonic circRNA, ecircRNA)。ecircRNA可充当竞争性内源RNA(ceRNA)结合天然微小RNA(miRNA)并阻断其对靶基因表达的抑制作用,作为miRNA的分子海绵发挥功能;(2)内含子配对驱动环化,为两个内含子的碱基互补配对形成环状结构,然后剪除内含子变成circRNA(circularintronic RNA, ciRNA);(3)同时包含外显子和内含子的circRNA(eIciRNA)[10]。
1.2 circRNA对肿瘤的生物调控机制
1.2.1 调控基因转录
circRNA在转录和转录后调控发挥作用。外显子源性的ecircRNA大多在细胞质中发挥作用,ciRNA及eIciRNA大多在细胞核中发挥作用,主要有两种途径:(1)ciRNA在细胞核内与RNA聚合酶Ⅱ(Pol-Ⅱ)结合促进其亲本基因表达;(2)eIciRNA在细胞核内与U1小核糖核蛋白(U1 snRNP)结合形成复合物,与Pol-Ⅱ转录复合物结合于亲本基因启动子上,促其亲本基因转录[11]。如circRNA基因敲除导致其亲本基因表达降低,位于转录位点附近的ci-ankyrd 52(ciRNA)是一种丰富的RNA,其重复结构域可以影响RNA-pol-Ⅱ复合物的延伸,是Pol-Ⅱ复合物的正调控因子,对其亲本基因编码具有顺式调控作用[12]。
1.2.2 circRNA作为miRNA分子海绵
circRNA作为miRNA海绵,参与肿瘤细胞的多种活动,包括肿瘤增殖、远处转移和化疗耐药性等。circRNA与下游靶基因mRNA竞争性结合miRNA,进而影响mRNA的基因表达,这也是circRNA目前研究最多的生物学机制[13],即为“circRNA/miRNA/mRNA”轴的调节,miRNAs通过与靶mRNA的3'-UTR结合发挥调控靶mRNA表达的作用,circRNA则通过海绵吸附miRNA,竞争性阻止或减少了miRNA与靶mRNA结合,从而在转录和翻译水平上调节了靶mRNA基因表达,促使细胞增殖、凋亡和恶变等,进而促进肿瘤发生发展[14]。
1.2.3 蛋白质翻译
通常circRNA是不能被翻译的,但随着研究深入,发现一些circRNA的外显子序列可被翻译成蛋白质。大部分包含有外显子circRNA与核糖体进入位点(internal ribosome entry site, IRES)结合后能在体内外进行翻译[15]。目前已有一些circRNA被证实是由IRES介导翻译,如circ-FBXW7、circ-MBl、circ-ZNF609等。除了经典翻译模式和上述IRES介导的翻译模式之外,N6-甲基腺苷(N6-methyladenosine, m6A)残基富集于circRNA上,作为IRES翻译circRNA[16]。METTL3是一种主要的RNA N6腺苷甲基转移酶,在人肝细胞癌和多发性实体瘤中显著上调,METTL3通过m6A-YTHDF2依赖机制抑制肝癌中SOCS2的表达进而加速HCC的进展[17]。还有一些circRNA能与蛋白质相互作用抑制翻译进程,WTAP通过m6A-HuR途径抑制ETS1的表达,从而促进HCC的进展[18]。circRNA参与蛋白质翻译在肝癌进展中转录后调控的作用机制仍未探明。circRNA参与蛋白质翻译及其在肿瘤和相关组织中的调控作用可能为circRNA进一步研究提供思路。
1.2.4 circRNA与结合蛋白相互作用
结合蛋白(RNA binding protein, RBP)是能够影响circRNA加工、折叠和定位的一类广泛的蛋白质[19]。有研究表明,circRNA可以作为RBP的海绵与RNA QKI、MBL、PolⅡ、Argonaute(AGO)、真核起始因子4A-Ⅲ(EIF4A3)等形成大的RNA-蛋白复合物(RPC),这些RPC可以调节RBP或miRNA的功能,进而调节亲本基因或相关基因的转录[20]。circRNA可以与RBP结合促进肿瘤的发展,circ-Ago2和HuR相互作用并被激活,促进癌细胞的生长、侵袭、转移,同时促进HuR在靶mRNA3'UTR上的富集,从而阻止Ago2与靶基因的结合,使Ago2/miRNA介导的基因受到抑制,进而促进肿瘤发生和侵袭[21]。此外,RBP还可以通过抑制circRNA的表达来促进癌细胞的增殖,RBP-RBM3的高表达会抑制SCD-circRNA2的表达,从而促进HCC细胞的增殖[22],见图 1。
![]() 图 1 环状RNA的形成与生物学功能Figure 1 Formation and biological function of circRNAThe formation mode of circRNA: Lasso-driven cyclization ecircRNA; Intron pairing drives cyclized ciRNA; eIcirRNA contains both exons and introns. CircRNA plays its biological functions by regulating gene transcription, acting as a molecular sponge of microRNA, participating in protein translation, and interacting with RNA binding protein(RBP).
图 1 环状RNA的形成与生物学功能Figure 1 Formation and biological function of circRNAThe formation mode of circRNA: Lasso-driven cyclization ecircRNA; Intron pairing drives cyclized ciRNA; eIcirRNA contains both exons and introns. CircRNA plays its biological functions by regulating gene transcription, acting as a molecular sponge of microRNA, participating in protein translation, and interacting with RNA binding protein(RBP).2 CircRNA在HCC发生发展中的作用
2.1 circRNA是HCC的致癌因子
circ-0061395在肝癌组织、血清、细胞和血清衍生外泌体中表达上调,在体外诱导细胞周期阻滞、凋亡,抑制增殖、侵袭和迁移。circ-0061395竞争性结合miR-877-5p,调节PIK3R3的表达促进HCC的生长[23]。外泌体circ-ZNF652通过miR-29a-3p/GUCD1转移到HCC细胞中,参与HCC细胞增殖、迁移、侵袭和糖酵解[24]。外泌体circ-100338增强HCC细胞的侵袭性和血管生成,促进HCC的转移[25]。circ-MAST1在HCC组织和细胞系中表达上调,circ-MAST1吸附miR-1299稳定CTNND1的表达,促进HCC细胞的生长[26]。circ-0021093作为ceRNA充当miR-432的海绵,促进HCC细胞的增殖、迁移和侵袭来促进HCC的进展[27]。hsa-circ-0000711在肝癌细胞中表达上调,并通过靶向has-miR-103a-3p促进肝癌细胞增殖、抑制凋亡[28]。circ-0004277在HCC细胞、组织和血浆外泌体中的表达明显上调,其过表达增强了HCC细胞在体内和体外的增殖、迁移和上皮-间质转化(EMT),加速HCC的进展。HCC细胞中的外泌体circ-0004277通过细胞交流刺激周边细胞的EMT,进一步促进HCC细胞对周围正常组织的侵袭,加速HCC的发展[29]。由此可见,有相当一部分circRNA作为HCC的促癌因子促进HCC的进展。
2.2 circRNA在HCC中作为抑癌因子
circ-MTO1在肝癌组织和细胞系中表达下调,circ-MTO1作为miR-9-5p的分子海绵,NOX4是miR-9-5p的靶基因。过表达的circ-MTO1和NOX4抑制HCC细胞的增殖和迁移并诱导其凋亡[30]。circ-0051443通过外泌体从正常细胞转运到HCC细胞,与miR-331-3p竞争性结合,诱导BAK1的表达,促进细胞凋亡和阻滞细胞周期抑制恶性生物学行为,抑制HCC的进展[31]。hsa-circ-0074854在HCC细胞中表达下调,通过与HuR相互作用与抑制外显体介导的巨噬细胞M2极化,抑制HCC细胞的迁移和侵袭[32]。circ-0001445在HCC表达下调,circ-0001445的过表达并海绵化miR-942-5p提高ALX4表达,抑制HCC细胞转移、EMT和糖酵解,并诱导细胞周期阻滞,circ-0001445可能成为抑制HCC发生发展的潜在靶点[33]。已有大量circRNA被鉴定为HCC的抑癌因子,在肝癌细胞组织中表达下调,未来有可能作为治疗靶点,在HCC早期进行干预,控制HCC进展。
2.3 circRNA与HCC相关肿瘤信号通路
circRNA通过吸附microRNA(miR)和破坏细胞信号通路参与HCC的发生发展。circRNA常与经典肿瘤信号通路相关靶蛋白关联密切。在一项针对肝癌样本的研究中,circ-PTGR1过表达激活Met信号通路,并海绵化miR-449a,参与细胞分化、抑制HCC细胞的侵袭和转移[34]。circZNF609抑制miR-15a-5p/15b-5p的表达、提高GLI2的表达,激活Hedgehog通路参与HCC细胞的增殖、侵袭和转移,促进HCC的进展[35]。circRNA-ITCH参与调节Wnt/RNA-ITCH促信号通路,抑制C-myc和cyclind1的表达,circRNA-ITCH通过抑制Wnt /NA-ITCH促进信号转导通路发挥致癌作用参与HCC进展[36]。circ-0014717抑制BTG2的表达,并通过海绵化miR-668-3p抑制HCC细胞的生长、迁移和侵袭。发现一个新的circ-0014717/miR-668-3p/BTG2相关HCC的信号调控通路[37]。has-circ-0007456在肝癌细胞系中的表达水平显著影响其对NK细胞的敏感度,实验验证hsa-circ-0007456通过吸附miR-6852-3p和ICAM-1的表达来发挥作用机制。miR-6852-3p/ICAM-1轴是has-circ-0007456介导的NK细胞杀伤HCC细胞所必需的。has-circ-0007456/miR-6852-3p/ICAM-1轴是肿瘤免疫逃逸和肝癌发生过程中的重要信号通路[38]。
除上述与HCC相关肿瘤信号通路外,还有许多具有不同效应的circRNA与HCC相关的经典信号通路有关,circRNA作为其中重要靶基因,参与HCC的发生发展,这些信号通路的激活或抑制下游致癌或抑癌靶基因、介导下游靶蛋白的表达发生变化,进而产生抑癌或促癌作用。HCC相关circRNA肿瘤信号通路机制复杂多样,circRNA在HCC的发生发展机制及临床诊治具有重大意义。
2.4 circRNA参与HCC肿瘤微环境
肿瘤微环境(tumor microenvironment, TME)被认为是肿瘤增殖、免疫逃逸、转移和化疗耐药的关键因素[39]。首先,circRNA可参与PD-L1调节肿瘤免疫逃逸,circRNA作为ceRNA调节PD-L1的表达,从而帮助肿瘤细胞逃避免疫监视[40]。在肝癌细胞中高表达的hsa-circ-0003288,通过PI3K/AKT信号通路作为miR-145的海绵,上调PD-L1的表达,引起肿瘤免疫逃逸,促进EMT和HCC细胞侵袭,发挥致癌作用,靶向has-circ-0003288可能是肝癌的一个有价值的治疗靶点[41];第二,缺氧是TME的典型特征,低氧的微环境可加强癌细胞对放化疗的抵抗性,对肿瘤侵袭和治疗有重要影响[42]。cZNF292在肝癌细胞中以时间依赖的方式诱导,而不依赖于缺氧诱导因子(HIF)-1α。cZNF292基因敲除增加了SRY-box 9(SOX9)核转位,降低了Wnt/β-catenin通路的活性,从而抑制了缺氧性肝癌细胞的增殖、VM和体外抗辐射性[43];第三,HCC是一种典型的富血管性肿瘤,内皮细胞作为肿瘤微环境的重要组成部分,参与血管生成,影响HCC的发生发展[29]。circ-4911和circ-4302抑制HCC微环境中人脐静脉内皮细胞(HUVECs)的增殖和迁移,进而抑制HCC的进展[44]。
此外,circRNA还可参与重塑细胞外基质(extracellular matrix,ECM)、调控血管生成等过程参与TME,在调节肝纤维化、肝癌发生、上皮间质转化、肿瘤侵袭和转移中发挥重要作用,并通过与肿瘤细胞的串扰在肿瘤相关疾病中发挥作用[31]。circRNA在TME中发挥多种作用,但具体机制研究仍处于起步阶段,在未来很可能作为一个热点机制探索。
3 circRNA在HCC中的潜在临床价值
circRNA在细胞质中具有进化保守、表达丰富、结构稳定等特性,在肿瘤诊断、治疗、判断预后等方面具有较大临床应用价值,可作为HCC的新型生物标志物和治疗靶点[45]。血浆circRNA面板(circPanel),其中包含三个检测HCC的circRNA(hsa-circ-0000976、hsa-circ-0007750和hsa-circ-0139897)联合应用。circPanel对HCC和小肝癌的诊断准确率优于AFP,能有效鉴别AFP阳性和AFP阴性的小肝癌。因此,研究者认为circPanel可作为肝癌临床诊断的一个新型生物标志物[46]。circRNA可以细胞特异性的方式广泛分布于血浆、尿液、组织样本、细胞唾液等人体成分中,检测标本较易获取,外周血和其他体液中等无创性标本检测的生物标志物具有较好的临床应用价值,将成为癌症筛查新型指标[47]。circRNA还可以预测HCC患者的生存。hsa-circ-0036683被发现是HCC的一个独立且重要的预后因子,并强调它作为预测患者生存的候选生物标志物具有潜在的临床实用价值[48]。HCC患者中circARPP21的表达降低与肿瘤体积增大、tug淋巴结转移(TNM)分期和甲胎蛋白(AFP)水平升高有关。研究验证circARPP21作为miR-543的海绵,通过调节LIFR抑制HCC细胞的增殖、存活、侵袭和迁移,延缓HCC的进展[49]。circRNA与HCC的进展和放化疗耐药有关。例如circ-0031242与肝癌对DDP的耐药性有关。circ-0031242的沉默降低了DDP耐药细胞(Huh7-R和SNU-387-R)的细胞活力、迁移、侵袭和凋亡,增强了DDP的体内敏感性。调控HCC的进展和DDP耐药影响[50]。
circRNAs调控肿瘤微环境、肿瘤免疫、化学耐药等的机制是目前研究的热点。因此,亟需探寻更多的靶向HCC的circRNA参与临床HCC的治疗和判断预后。随着相关cirRNA与HCC机制和功能被探明,期待在众多circRNA中探索出靶向HCC的circRNA作为新型生物标志物参与临床诊断、治疗、判断预后,见表 1。
表 1 HCC发生发展相关的circRNATable 1 CircRNA related to occurrence and development of HCC
4 总结与展望
circRNA作为HCC新型标志物,在HCC的早期诊断、治疗、预后判断中具有潜在临床价值。circRNA的异常表达通过调控基因转录、作为miRNA海绵、参与蛋白质翻译、与RBP相互作用等发挥生物学机制,参与调控HCC细胞的发生、发展、侵袭和转移。当前的研究主要集中在其表达改变和作为miRNA海绵的吸附作用机制上,对于circRNA蛋白质翻译和调控基因表达的具体分子机制尚不明确。大部分circRNAs与其亲本基因、RNA结合蛋白等的作用机制关系亦不清楚。随着基础研究的不断深入,诸多与肿瘤相关的circRNA被发现,目前研究中广泛存在的问题是circRNA的命名还不够规范,亟需建立circRNA的通用标准和规范。circRNA在组织和体液中具有较高的特异性和稳定性,标本也较易获得,由于circRNA与肿瘤类型还不能一一对应为特异性指标,故而组合circPanel(多个对HCC有特异性的circRNA组合)有望成为HCC新型早期诊断、预后评价和潜在治疗靶点。
Competing interests: The authors declare that they have no competing interests.利益冲突声明:所有作者均声明不存在利益冲突。作者贡献:任文镇:研究设计、资料收集、统计分析、论文撰写王宏浩:文献查阅、统计分析向 田:资料收集、文献查阅刘 杲:研究设计、论文审阅、论文修改 -
表 1 LNR、SII、PLR与胃癌临床特征相关分析(n(%))
Table 1 Correlation of LNR, SII, and PLR with clinical features of gastric cancer patients (n(%))

表 2 NLR、LMR、OPNI与胃癌临床特征相关分析(n(%))
Table 2 Correlation of NLR, LMR, and OPNI with clinical features of gastric cancer patients (n(%))

表 3 胃癌总生存期影响因素的Cox单因素和多因素分析
Table 3 Cox univariate and multivariate analyses of influencing factors for overall survival of gastric cancer patients

-
[1] Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. doi: 10.3322/caac.21660
[2] Li P, Chen H, Chen S, et al. Circular RNA 0000096 affects cell growth and migration in gastric cancer[J]. Br J Cancer, 2017, 116(5): 626-633. doi: 10.1038/bjc.2016.451
[3] Mollinedo F. Neutrophil Degranulation, Plasticity, and Cancer Metastasis[J]. Trends Immunol, 2019, 40(3): 228-242. doi: 10.1016/j.it.2019.01.006
[4] Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis[J]. J Hematol Oncol, 2021, 14(1): 173. doi: 10.1186/s13045-021-01187-y
[5] Zare Moayedi M, Ahmmadpour F, Rashidi M, et al. The association between mRNA expression of resistin, TNF-α, IL-6, IL-8, and ER-α in peripheral blood mononuclear cells and breast cancer[J]. Turk J Med Sci, 2021, 51(3): 1345-1353. doi: 10.3906/sag-2008-292
[6] Hirahara T, Arigami T, Yanagita S, et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer[J]. BMC cancer, 2019, 19(1): 672. doi: 10.1186/s12885-019-5903-y
[7] Trinh H, Dzul S P, Hyder J, et al. Prognostic value of changes in neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte-to-monocyte ratio (LMR) for patients with cervical cancer undergoing definitive chemoradiotherapy (dCRT)[J]. Clin Chim Acta, 2020, 510: 711-716. doi: 10.1016/j.cca.2020.09.008
[8] Xia LJ, Li W, Zhai JC, et al. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio and prognostic nutritional index for predicting clinical outcomes in T1-2 rectal cancer[J]. BMC cancer, 2020, 20(1): 208. doi: 10.1186/s12885-020-6698-6
[9] Huang Y, Gao Y, Wu Y, et al. Prognostic value of systemic immune-inflammation index in patients with urologic cancers: a meta-analysis[J]. Cancer Cell Int, 2020, 20: 499. doi: 10.1186/s12935-020-01590-4
[10] Cheng H, Luo G, Lu Y, et al. The combination of systemic inflammation-based marker NLR and circulating regulatory T cells predicts the prognosis of resectable pancreatic cancer patients[J]. Pancreatology, 2016, 16(6): 1080-1084. doi: 10.1016/j.pan.2016.09.007
[11] Liu R, Dai T, Zheng S, et al. Prognostic value of combined pretreatment fibrinogen and neutrophil-lymphocyte ratio in digestive system cancers: a meta-analysis of 17 retrospective studies[J]. Transl Cancer Res, 2021, 10(1): 241-250. doi: 10.21037/tcr-20-2482
[12] Huang Z, Chen Y, Zhang W, et al. Modified Gastric Cancer AJCC Staging with a Classification Based on the Ratio of Regional Lymph Node Involvement: A Population-Based Cohort Study[J]. Ann Surg Oncol, 2020, 27(5): 1480-1487. doi: 10.1245/s10434-019-08098-w
[13] Agnes A, Biondi A, Cananzi FM, et al. Ratio-based staging systems are better than the 7th and 8th editions of the TNM in stratifying the prognosis of gastric cancer patients: A multicenter retrospective study[J]. J Surg Oncol, 2019, 119(7): 948-957. doi: 10.1002/jso.25411
[14] Sakin A, Atci MM, Aldemir MN, et al. The Prognostic Value of Postoperative Lymph Node Ratio in Gastric Adenocarcinoma Patients Treated With Neoadjuvant Chemotherapy[J]. Cureus, 2021, 13(4): e14639. http://pubmed.ncbi.nlm.nih.gov/34046274/
[15] Stoltzfus CR, Sivakumar R, Kunz L, et al. Multi-Parameter Quantitative Imaging of Tumor Microenvironments Reveals Perivascular Immune Niches Associated With Anti-Tumor Immunity[J]. Front Immunol, 2021, 12: 726492. doi: 10.3389/fimmu.2021.726492
[16] Singh A, Anang V, Kumari K, et al. Role of lymphocytes, macrophages and immune receptors in suppression of tumor immunity[J]. Prog Mol Biol Transl Sci, 2023, 194: 269-310.
[17] Yu K, Qiang G, Peng S, et al. Potential diagnostic value of the hematological parameters lymphocyte-monocyte ratio and hemoglobin-platelet ratio for detecting colon cancer[J]. J Int Med Res, 2022, 50(9): 3000605221122742.
[18] Hajizadeh F, Aghebati Maleki L, Alexander M, et al. Tumor-associated neutrophils as new players in immunosuppressive process of the tumor microenvironment in breast cancer[J]. Life Sci, 2021, 264: 118699. doi: 10.1016/j.lfs.2020.118699
[19] Wang H, Zhang B, Li R, et al. KIAA1199 drives immune suppression to promote colorectal cancer liver metastasis by modulating neutrophil infiltration[J]. Hepatology, 2022, 76(4): 967-981. doi: 10.1002/hep.32383
[20] Huang Y, Chen Y, Zhu Y, et al. Postoperative Systemic Immune-Inflammation Index (SⅡ): A Superior Prognostic Factor of Endometrial Cancer[J]. Front Surg, 2021, 8: 704235. doi: 10.3389/fsurg.2021.704235
[21] Zahorec R. Neutrophil-to-lymphocyte ratio, past, present and future perspectives[J]. Bratisl Lek Listy, 2021, 122(7): 474-488.
[22] Jiang Y, Xu D, Song H, et al. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: a systematic review and meta-analysis[J]. BMJ Open, 2021, 11(9): e048324. doi: 10.1136/bmjopen-2020-048324
[23] Swierczak A, Mouchemore KA, Hamilton JA, et al. Neutrophils: important contributors to tumor progression and metastasis[J]. Cancer Metastasis Rev, 2015, 34(4): 735-751. doi: 10.1007/s10555-015-9594-9
[24] Liang W, Ferrara N. The Complex Role of Neutrophils in Tumor Angiogenesis and Metastasis[J]. Cancer Immunol Res, 2016, 4(2): 83-91. doi: 10.1158/2326-6066.CIR-15-0313
[25] Zahorec R. Ratio of neutrophil to lymphocyte counts-rapid and simple parameter of systemic inflammation and stress in critically ill[J]. Bratisl Lek Listy, 2001, 102(1): 5-14. http://bmj.fmed.uniba.sk/2001/10201-01.PDF
[26] el-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system[J]. J Immunol, 1987, 139(7): 2406-2413. doi: 10.4049/jimmunol.139.7.2406
[27] Petrie HT, Klassen LW, Kay HD. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes[J]. J Immunol, 1985, 134(1): 230-234. doi: 10.4049/jimmunol.134.1.230
[28] Tan KW, Chong SZ, Wong FHS, et al. Neutrophils contribute to inflammatory lymphangiogenesis by increasing VEGF-A bioavailability and secreting VEGF-D[J]. Blood, 2013, 122(22): 3666-3677. doi: 10.1182/blood-2012-11-466532
[29] Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis[J]. J Natl Cancer Inst, 2014, 106(6): dju124. http://jnci.oxfordjournals.org/content/106/6/dju124.full.pdf
[30] Fang T, Wang Y, Yin X, et al. Diagnostic Sensitivity of NLR and PLR in Early Diagnosis of Gastric Cancer[J]. J Immunol Res, 2020, 2020: 9146042. http://www.xueshufan.com/publication/3009855148
[31] Wang H, Ding Y, Li N, et al. Prognostic Value of Neutrophil-Lymphocyte Ratio, Platelet-Lymphocyte Ratio, and Combined Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio in Stage Ⅳ Advanced Gastric Cancer[J]. Front Oncol, 2020, 10: 841. doi: 10.3389/fonc.2020.00841
[32] 陈奕心, 孙轶华, 程洋洋, 等. 术前炎症相关指标与可切除胃癌患者预后的相关[J]. 实用肿瘤学杂志, 2021, 35(6): 517-522. https://www.cnki.com.cn/Article/CJFDTOTAL-SYZL202106008.htm Chen YX, Sun YH, Cheng YY, et al. Relationship between preoperative inflammation-related indicators and prognosis of patients with resectable gastric cancer[J]. Shi Yong Zhong Liu Xue Za Zhi, 2021, 35(6): 517-522. https://www.cnki.com.cn/Article/CJFDTOTAL-SYZL202106008.htm
[33] Mungan İ, Dicle Ç B, Bektaş Ş, et al. Does the preoperative platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio predict morbidity after gastrectomy for gastric cancer?[J]. Mil Med Res, 2020, 7: 9. http://www.xueshufan.com/publication/3018592043
[34] Miyamoto R, Inagawa S, Sano N, et al. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients[J]. Eur J Surg Oncol, 2018, 44(5): 607-612. doi: 10.1016/j.ejso.2018.02.003
[35] Wang J, Dang P, Raut CP, et al. Comparison of a lymph node ratio-based staging system with the 7th AJCC system for gastric cancer: analysis of 18, 043 patients from the SEER database[J]. Ann Surg, 2012, 255(3): 478-485. doi: 10.1097/SLA.0b013e31824857e2
[36] Liu YY, Fang WL, Wang F, et al. Does a Higher Cutoff Value of Lymph Node Retrieval Substantially Improve Survival in Patients With Advanced Gastric Cancer?-Time to Embrace a New Digit[J]. Oncologist, 2017, 22(1): 97-106. doi: 10.1634/theoncologist.2016-0239
-
期刊类型引用(3)
1. 刘丽琼. miRNA与肝细胞癌的相关性研究进展. 生物化工. 2024(02): 219-222 .  百度学术
百度学术
2. 徐藓寓,朱永平,刘艳青,谷丽维,张珺哲,沈胜楠,王继刚. 中医药基于表观遗传调控肝细胞癌的作用机制研究进展. 中国实验方剂学杂志. 2024(23): 281-291 .  百度学术
百度学术
3. 赖思思,付艳丽,张伊,黄海福. 浅析非编码RNA在肝癌中的研究现状. 中医肿瘤学杂志. 2023(04): 50-57 .  百度学术
百度学术
其他类型引用(1)




 下载:
下载: