-
摘要:目的
了解目前肺癌免疫治疗领域的研究现状,以期为该领域深入研究和未来选题提供一定参考。
方法CiteSpace可视化分析软件对2005—2021年中国知网(CNKI)收录的以“肺癌”和“免疫治疗”为关键词的400篇中文文献,以及Web of Science数据库收录的5001篇英文文献进行可视化分析。同时对Web of Science数据库收录的“Lung Cancer”和“Immunotherapy”和“Single cell sequencing”17篇英文文献进行关键词共现分析。
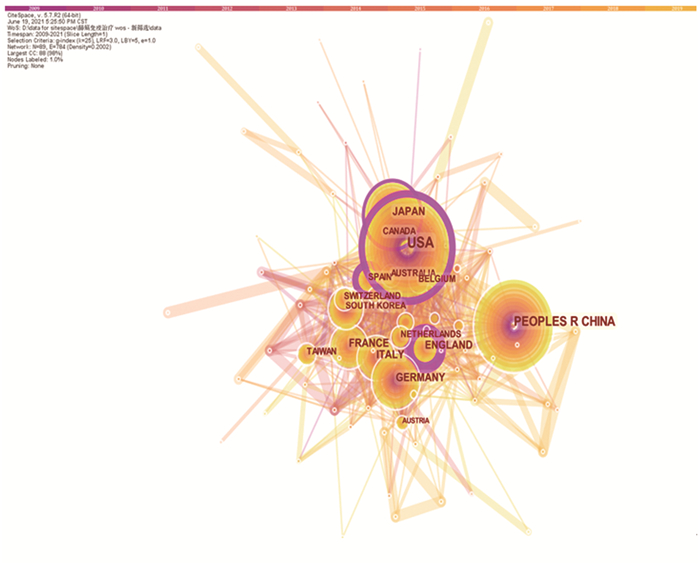
结果“非小细胞肺癌”、“免疫抑制剂”、“PD-L1”、“树突状细胞”、“细胞因子诱导的杀伤细胞”等是目前肺癌免疫治疗研究的热点。单抗药物以纳武单抗(Nivolumab)、帕博利珠单抗(Pembrolizumab)、阿特珠单抗(Atezolizumab)和德瓦鲁单抗(Durvalumab)为热点药物。免疫治疗与化疗联合治疗、PD-L1的表达相关的研究也成为持续研究重点。肺癌免疫治疗研究在美国最多,其次为中国。
结论目前我国对肺癌免疫治疗相关研究逐渐成为热点,未来需对当前研究热点进行更加深入的研究,为肺癌免疫治疗提供更为前沿的方向。
Abstract:ObjectiveTo understand the current status of research on lung cancer immunotherapy to provide a reference for further investigation and future topic selection in this field.
MethodsCiteSpace visualization analysis software was used to analyze 400 Chinese studies in CNKI and 5 001 English studies in the Web of Science database from 2005 to 2021, with "lung cancer" and "immunotherapy" as keywords. Keyword co-occurrence analysis was performed on 17 English studies of "Lung Cancer" "Immunotherapy" and "Single cell sequencing" in the Web of Science database.
Results"Non-small cell lung cancer" "immunosuppressants" "PD-L1" "dendritic cells" and "cytokine-induced killer cells" are current research hotspots in lung cancer immunotherapy. Monoclonal antibody drugs including nivolumab, pembrolizumab, atezolizumab, and durvalumab are hotspot drugs. Immunotherapy combined with chemotherapy as well as PD-L1 expression have become the focus of continuous research. The majority of studies on lung cancer immunotherapy are conducted in the United States, followed by China.
ConclusionLung cancer immunotherapy has gradually become a research hot spot in China. In the future, in-depth research is needed to provide cutting-edge directions for lung cancer immunotherapy.
-
Key words:
- Lung cancer /
- Immunotherapy /
- Single cell sequencing /
- Visual analysis /
- CiteSpace
-
0 引言
膀胱癌(bladder cancer, BC)是中国泌尿生殖系统常见的恶性肿瘤之一[1],约75%新诊断的BC为非肌层浸润性膀胱癌(non-muscle invasive bladder cancer, NMIBC),局限于膀胱壁黏膜或黏膜下层,主要治疗手段为经尿道膀胱肿瘤切除术(transurethral resection of the bladder tumor, TURBT)联合膀胱内灌注化疗药物。但TURBT术后1年肿瘤复发率为40%~80%,25%的患者进展为肌层浸润性膀胱癌(muscle invasive bladder cancer, MIBC)[2]。目前普通白光膀胱镜检查是BC诊断和术后随访的标准技术手段,其对微小肿瘤、原位癌、术中残留肿瘤的识别能力有限,导致肿瘤的漏诊,而常规膀胱内灌注药物的不良反应严重影响患者术后生活质量[3]。因此,如何精准地发现病灶及界定肿瘤的边界、既能彻底切除肿瘤又能减少正常组织细胞的损伤成为了BC临床诊疗亟需解决的问题。
随着BC靶向分子载体、靶向特异性作用机制、靶向光学分子影像和靶向光免疫疗法(photoimmunotherapy, PIT)研究的不断深入,BC的靶向诊疗有望取得新的进展。为此,本文结合国内外最新文献及本研究组的前期工作,将已发现的BC靶向分子载体的研究现状综述如下。
1 靶向基团的种类及特性
靶向分子载体是指能够特异识别抗原蛋白并与之紧密结合的靶向基团,主要包括抗体、抗体片段、支架蛋白、肽和小分子等,见表 1[4]。抗体与抗原特异性结合以及成熟的抗体制备技术,使其成为临床应用最多的一类靶向基团,但单克隆抗体分子量较大(约150 kDa)、肾脏不能排泄、体内主要通过肝脏分解代谢,导致抗体具有血液内循环时间长、非特异性组织蓄积和组织穿透能力差等缺点[5-6]。而采用化学技术制备的抗体片段(微抗体、抗原结合片段、双抗体、单链可变片段等),分子量约为15~80 kDa,既保留了完整抗体特异性结合抗原的生物学特性,又具有组织渗透性强和血液滞留时间短的优点[7]。
另外,最新关注的靶向基团还有非免疫球蛋白类支架蛋白(scaffolds protein),如亲和体、结蛋白和Centyrin等[8-10],这些蛋白支架既有类似抗体的靶向功能,又具有相对分子量小、高亲和力、折叠速率快、理化性能稳定、能接受化学修饰等优点。
此外,肽类和小分子的分子量最小,能与肿瘤特异性结合,而不与或很少与正常组织、细胞结合的小分子肽被称为肿瘤导向肽(tumor homing peptide, THP)。THP为基础的载体系统能够有效穿透组织且几乎无免疫原性,其半衰期短、肾清除率快、组织蓄积少以及生产工艺便捷、成本更低等优势[11],具有良好的应用前景。
2 膀胱癌靶向分子载体
2.1 抗体及其衍生物
CD47是一种普遍存在于人类细胞膜表面的跨膜糖蛋白,与巨噬细胞表面的信号调节蛋白α结合后能抑制其吞噬功能使肿瘤产生免疫逃逸[12]。CD47在80%以上的BC细胞膜上高表达,包括乳头状尿路上皮癌、鳞状细胞癌、微乳头状瘤及腺癌[13]。本项目组使用Alexa Fluor 790标记的抗-CD47孵育26例新鲜离体人BC组织进行近红外荧光成像,肿瘤组织的平均荧光强度明显高于临近正常组织(分别为132.31±6.67和52.27±12.09, P<0.001),见图 1[14]。Pan等[13]评价了抗-CD47-Qdot625介导的内镜分子成像检测BC的诊断准确性,21例新鲜完整膀胱标本经抗-CD47-Qdot625孵育后,在蓝光下检测到119个可疑区域,通过病理学验证,其诊断敏感度和特异性分别为82.9%和90.5%。
![]() 图 1 膀胱癌组织图像Figure 1 Images of bladder cancer tissuesA: The image of tumor specimen under white light; B: After anti-CD47-Alexa Fluor 790 incubating, the image of tumor specimen under NIR light; C: After anti-CD47-Alexa Fluor 790 incubating, the pseudo-color image of fresh integrated tumor specimen under NIR light; D: The HE staining image of fresh integrated tumor specimen; E: The HE staining representative image of tumor area; NIR: near-infrared.
图 1 膀胱癌组织图像Figure 1 Images of bladder cancer tissuesA: The image of tumor specimen under white light; B: After anti-CD47-Alexa Fluor 790 incubating, the image of tumor specimen under NIR light; C: After anti-CD47-Alexa Fluor 790 incubating, the pseudo-color image of fresh integrated tumor specimen under NIR light; D: The HE staining image of fresh integrated tumor specimen; E: The HE staining representative image of tumor area; NIR: near-infrared.CAⅨ是碳酸酐酶家族中的一员,在缺氧条件下调节细胞内pH值,进而改变肿瘤细胞的黏附、增殖和进展的生物学特性[15]。免疫组织化学染色显示CAⅨ在BC组织中的阳性率高达67.1%,在正常的尿路上皮和膀胱慢性炎性病变组织中均呈阴性(P<0.01)[16]。Wang等[17]在8例新鲜完整膀胱标本内灌注抗-CAⅨ-Qdot625,白光膀胱镜诊断BC的敏感度为76.0%,特异性为90.5%,而抗-CAⅨ介导的蓝光内镜分子成像下的肿瘤检测获得了较高的诊断准确率,总体敏感度和特异性分别为88.00%和93.75%。
PIT是一种新型的分子靶向光动力治疗模式,亲水性酞菁染料IR700与单克隆抗体结合进行的靶向性PIT,有效减少了单纯IR700光动力疗法的不良反应[18]。Kiss等[19]研究发现,抗-CD47-IR700介导的PIT治疗,IR700使用剂量低,但显著增加了人膀胱肿瘤细胞系和原代膀胱肿瘤细胞的细胞毒作用。通过尾静脉注射抗-CD47-IR700对BC异种移植小鼠模型进行近红外光免疫治疗(NIR-PIT),单次治疗发现NIR-PIT组肿瘤生长明显减慢,在连续5周的治疗后,NIR-PIT能够有效抑制肿瘤生长(P=0.0104),明显延长治疗组小鼠的生存期(P=0.009)[20]。
单链可变片段(single-chain variable fragment, scFv)是抗体内部结合抗原的最小功能结构域,Rezaei等[20]开发了新型抗-CD47-scFv磁性纳米粒子(magnetic nanoparticles, MNP),体外研究表明抗-CD47-scFv-MNP对BC细胞系EJ138和5637具有高亲和力,经外磁场作用的靶向热疗后能显著降低肿瘤细胞存活率。
2.2 肿瘤导向肽(THP)
目前,利用噬菌体展示肽库技术和组合化学方法,先后筛选出四种与BC结合的靶向配体,分别是九肽序列Bld-1[21]、环九肽序列PLZ4[22]、环七肽序列NYZL1[23]和PLSWT7[24]。其中前两种筛选自MIBC细胞系,后两种则来自NMIBC细胞系。研究显示,四种THP均能在离体细胞、组织和小鼠体内与BC特异性结合,但它们结合肿瘤细胞的分子位点目前还不清楚[21-24]。
PLSWT7是目前唯一运用于内镜下分子成像的多肽载体。Peng等[24]将PLSWT7-IRDye800CW分子探针灌注入8例离体人膀胱腔内,分析了40个膀胱内感兴趣区域,进行了近红外分子成像诊断与组织病理学比较研究,发现PLSWT7-IRDye800CW分子影像诊断BC的敏感度和特异性分别为84.00%和86.70%。
常规尿液脱落细胞检查是一种简单易行的非侵入性诊断方法,但检测的敏感度较低。Jia等[25]使用Bld-1-FITC探针结合80例血尿患者尿液中的肿瘤细胞,其诊断BC的敏感度和特异性分别为79.31%和100%,优于尿脱落细胞学检查和荧光原位杂交技术检查(敏感度分别为20.69%和72.41%)。另外一项包含66例BC的研究发现,NYZL1-FITC探针与尿液中肿瘤细胞的结合与肿瘤恶性程度成正比,平均阳性结合百分比在Ta、T1、T2和T3~T4期分别为30%、57%、73%和85%[26]。
在靶向化疗方面,Bld-1能够与凋亡肽KLA和阿霉素(DOX)构建靶向制剂,并内化进入人膀胱癌HT1376细胞[27-28]。Jung等[27]评价了Bld-1-KLA在小鼠体内的治疗效能,静脉注射给药4周后,Bld-1-KLA组小鼠的肿瘤体积明显小于对照组(P<0.001)。Wei等[28]使用Bld-1-DOX治疗荷瘤小鼠16天后,Bld-1-DOX组的肿瘤体积明显缩小,且小鼠无明显不良反应,而单用DOX治疗组出现明显的心脏和肝脏损害。Lin等[29]开发了PLZ4靶向纳米胶束,在靶向递送方面的效率是非靶向胶束的1.5倍(P<0.05)且是游离染料的14.3倍(P<0.01),靶向胶束不仅能黏附在肿瘤细胞表面,还能被靶细胞摄取。使用PLZ4纳米胶束介导的紫杉醇(PTX)进行靶向化疗,成功将荷瘤小鼠的中位存活期从单纯紫杉醇治疗组的55天提高到了靶向治疗组的69.5天(P=0.03)[30]。
术中光学分子影像引导的外科手术被认为是继开放手术、微创外科手术之后的第三代外科手术新模式。传统的依据白光内窥镜图像和操作者的经验确定肿瘤浸润深度和手术切缘的方式具有主观性。一项涉及8 490例经TURBT治疗BC患者的系统回顾显示,17%~67%的初发Ta期患者和20%~71%的初发T1期患者在复发时发现了残留肿瘤,36%~86%残留肿瘤位于初次切除部位[3]。Peng[24]等采用PLSWT7-IRDye800CW进行了分子成像引导BC手术治疗的临床前研究,将小鼠异种移植模型随机分为两组:对照组(n=20)和实验组(n=20)分别在自然光和光学分子成像下进行肿瘤切除术。1周后,对照组和实验组肿瘤复发率分别为95%和5%;术后30天两组的总存活率分别为0和90%。
3 膀胱癌的多载体靶向和多模态诊疗
BC具有较强的异质性,单纯的一种靶向分子载体很难使全部患者受益,使用抗-CD47、抗-CAⅨ和无靶向性IgG4三种单抗对离体BC组织进行多路复合分子成像发现,诊断图像较单靶点成像噪声更低,分辨率更加精准(ROC AUC为0.93(0.73, 1.0))[31]。多模态分子探针能集成多种成像和(或)治疗模式,Lin等[32]开发的PLZ4-纳米卟啉平台,在进行BC光动力学诊断的同时实现BC的靶向光动力学治疗、靶向光热治疗和靶向化疗结合的三模态治疗,显著提升BC的临床诊断水平和治疗水平。
4 展望与挑战
综上所述,靶向分子载体介导的靶向性定位功能在膀胱癌早期诊断、完整切除、靶向治疗等方面具有巨大的应用潜力,但仍有许多科学问题和技术难点需要解决,比如靶向载体、连接体、荧光染料等的安全性验证,临床有效性的标准化评价体系的建立等,需要临床医生、化学家、物理学家、药理学家和工程师之间的多学科交叉协作。
Competing interests: The authors declare that they have no competing interests.作者贡献:杨淑燕:数据检索分析、图表绘制、论文撰写及修改庄金满、刘宇航:数据检索朱金秀、林梦心:指导数据分析何斐:论文指导和审核 -
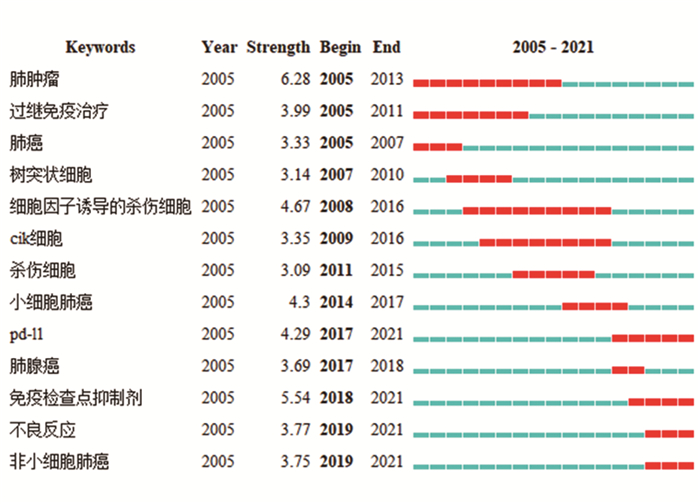
表 1 中国知网中肺癌免疫治疗前十位关键词
Table 1 Top ten keywords of lung cancer immunotherapy in CNKI

表 2 中国知网中肺癌免疫治疗聚类分析情况列表
Table 2 List of cluster analysis of lung cancer immunotherapy in CNKI

表 3 纳入的Web of Science英文肺癌免疫治疗文章被引次数最多的10篇文献
Table 3 Top 10 most cited English articles on lung cancer immunotherapy in the Web of Science database

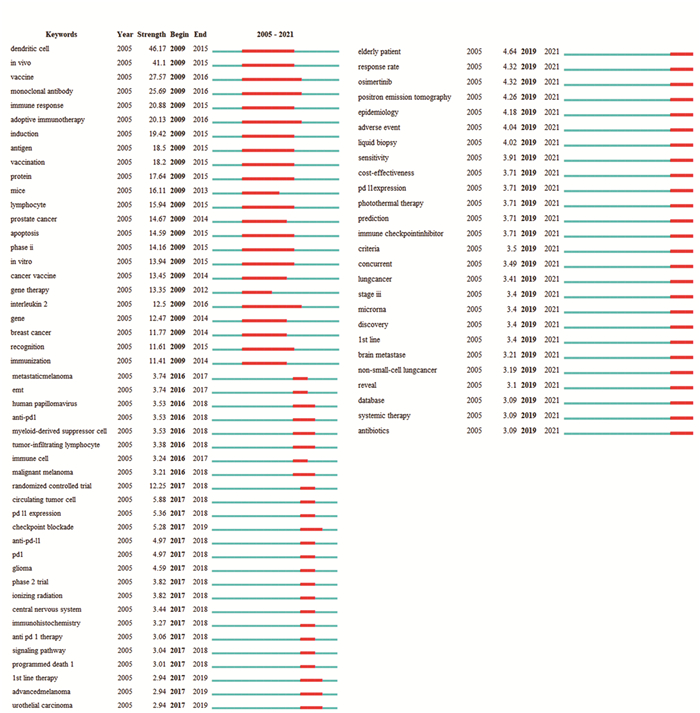
表 4 纳入的Web of Science数据库中英文肺癌免疫治疗文章出现的前十位关键词
Table 4 Top 10 keywords in English articles on lung cancer immunotherapy in the Web of Science database

表 5 纳入的Web of Science数据库中英文肺癌免疫治疗文章关键词聚类信息
Table 5 Keyword clustering information of English articles on lung cancer immunotherapy in the Web of Science database

表 6 单细胞测序技术在肺癌免疫治疗中的关键词
Table 6 Keywords of single-cell sequencing technology in lung cancer immunotherapy

-
[1] Sundar R, Soong R, Cho BC, et al. Immunotherapy in the treatment of non-small cell lung cancer[J]. Lung Cancer, 2014, 85(2): 101-109. doi: 10.1016/j.lungcan.2014.05.005
[2] 王丽萍. 肺癌免疫治疗现状及展望[J]. 中华实用诊断与治疗杂志, 2017, 31(2): 105-110. doi: 10.13507/j.issn.1674-3474.2017.02.001 Wang LP. Current Situation and Prospect of Immunotherapy for Lung Cancer[J]. Zhonghua Shi Yong Zhen Duan Yu Zhi Liao Za Zhi, 2017, 31(2): 105-110. doi: 10.13507/j.issn.1674-3474.2017.02.001
[3] Konala VM, Madhira BR, Ashra S, et al. Use of Immunotherapy in Extensive-Stage Small Cell Lung Cancer[J]. Oncology, 2020, 98(11): 749-754. doi: 10.1159/000508516
[4] Ko EC, Raben D, Formenti SC, et al. The Integration of Radiotherapy with Immunotherapy for the Treatment of Non-Small Cell Lung Cancer[J]. Clin Cancer Res, 2018, 24(23): 5792-5806. doi: 10.1158/1078-0432.CCR-17-3620
[5] Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer[J]. N Engl J Med, 2018, 379(23): 2220-2229. doi: 10.1056/NEJMoa1809064
[6] 孙凤环, 陈健, 张鹏. 单细胞测序在肿瘤基因检测中的研究进展[J]. 同济大学学报(医学版), 2019, 40(1): 117-122. doi: 10.16118/j.1008-0392.2019.01.022 Sun FH, Chen J, Zhang P. Research progress of single-cell sequencing in tumor gene detection[J]. Tongji Da Xue Xue Bao(Yi Xue Ban), 2019, 40(1): 117-122. doi: 10.16118/j.1008-0392.2019.01.022
[7] Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer[J]. N Engl J Med, 2015, 373(17): 1627-1639. doi: 10.1056/NEJMoa1507643
[8] Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer[J]. N Engl J Med, 2015, 373(2): 123-135. doi: 10.1056/NEJMoa1504627
[9] Reck M, Rodríguez-Abreu D, Robinson AG, et al. KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer[J]. N Engl J Med, 2016, 375(19): 1823-1833. doi: 10.1056/NEJMoa1606774
[10] Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial[J]. Lancet, 2016, 387(10027): 1540-1550. doi: 10.1016/S0140-6736(15)01281-7
[11] Garon EB, Rizvi NA, Hui R, et al. KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer[J]. N Engl J Med, 2015, 372(21): 2018-2028. doi: 10.1056/NEJMoa1501824
[12] Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer[J]. Science, 2015, 348(6230): 124-128. doi: 10.1126/science.aaa1348
[13] Rittmeyer A, Barlesi F, Waterkamp D, et al. OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial[J]. Lancet, 2017, 389(10066): 255-265. doi: 10.1016/S0140-6736(16)32517-X
[14] Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer[J]. N Engl J Med, 2012, 366(26): 2443-2454. doi: 10.1056/NEJMoa1200690
[15] Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients[J]. Nature, 2014, 515(7528): 563-567. doi: 10.1038/nature14011
[16] Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial[J]. Lancet, 2016, 387(10030): 1837-1846. doi: 10.1016/S0140-6736(16)00587-0
[17] 马晓骉, 罗峰, 高永亮, 等. CIK细胞免疫疗法联合化疗治疗非小细胞肺癌的临床疗效[J]. 昆明医科大学学报, 2020, 41(12): 60-67. https://www.cnki.com.cn/Article/CJFDTOTAL-KMYX202012012.htm Ma XB, Luo F, Gao YL, et al. Clinical efficacy of CIK cell immunotherapy combined with chemotherapy in the treatment of non-small cell lung cancer[J]. Kunming Yi Ke Da Xue Xue Bao, 2020, 41(12): 60-67. https://www.cnki.com.cn/Article/CJFDTOTAL-KMYX202012012.htm
[18] Wang L, Ma Q, Yao R, et al. Current status and development of anti-PD-1/PD-L1 immunotherapy for lung cancer[J]. Int Immunopharmacol, 2020, 79: 106088. doi: 10.1016/j.intimp.2019.106088
[19] 程颖. 晚期非小细胞肺癌免疫治疗的研究进展[J]. 肿瘤防治研究, 2021, 48(8): 745-750. doi: 10.3971/j.issn.1000-8578.2021.21.0472 Cheng Y. Research Progress of Immunotherapy for Advanced Non-small Cell Lung Cancer[J]. Zhong Liu Fang Zhi Yan Jiu, 2021, 48(8): 745-750. doi: 10.3971/j.issn.1000-8578.2021.21.0472
[20] 王奎, 张宏毅, 王泽权, 等. PD-1和PD-L1抑制剂治疗肺癌及其不良反应的研究现状及进展[J]. 中国医药科学, 2021, 11(3): 45-48. doi: 10.3969/j.issn.2095-0616.2021.03.012 Wang K, Zhang HY, Wang ZQ, et al. Research status and progress of PD-1 and PD-L1 inhibitors in the treatment of lung cancer and its adverse reactions[J]. Zhongguo Yi Yao Ke Xue, 2021, 11(3): 45-48. doi: 10.3969/j.issn.2095-0616.2021.03.012
[21] 付烊, 朱波, 章必成. 晚期肺癌免疫治疗的现状与未来[J]. 医药导报, 2019, 38(8): 993-996. https://www.cnki.com.cn/Article/CJFDTOTAL-YYDB201908004.htm Fu Y, Zhu B, Zhang BC. Current status and future of immunotherapy for advanced lung cancer[J]. Yi Yao Dao Bao, 2019, 38(8): 993-996. https://www.cnki.com.cn/Article/CJFDTOTAL-YYDB201908004.htm
[22] 张秀梅, 翟运开, 赵杰, 等. 肺癌靶向治疗研究: 以文献计量学和可视化分析述的热点与趋势[J]. 现代肿瘤医学, 2020, 28(19): 3417-3427. doi: 10.3969/j.issn.1672-4992.2020.19.031 Zhang XM, Zhai YK, Zhao J, et al. Lung cancer targeted therapy research: hotspots and trends described by bibliometrics and visual analysis[J]. Xian Dai Zhong Liu Yi Xue, 2020, 28(19): 3417-3427. doi: 10.3969/j.issn.1672-4992.2020.19.031
[23] Ding Z, Li Q, Zhang R, et al. Personalized neoantigen pulsed dendritic cell vaccine for advanced lung cancer[J]. Signal Transduct Target Ther, 2021, 6(1): 26. doi: 10.1038/s41392-020-00448-5
[24] 周莹, 黄华艺. 单细胞测序技术及其在肿瘤研究和临床诊断中的应用[J]. 分子诊断与治疗杂志, 2017, 9(3): 147-153, 172. doi: 10.3969/j.issn.1674-6929.2017.03.001 Zhou Y, Huang HY. Single-cell sequencing technology and its application in tumor research and clinical diagnosis[J]. Fen Zi Zhen Duan Yu Zhi Liao Za Zhi, 2017, 9(3): 147-153, 172. doi: 10.3969/j.issn.1674-6929.2017.03.001
[25] Eberwine J, Sul JY, Bartfai T, et al. The promise of single -cell sequencing[J]. Nat Methods, 2014, 11(1): 25-27. doi: 10.1038/nmeth.2769
[26] Borcoman E, Kanjanapan Y, Champiat S, et al. Novel patterns of response under immunotherapy[J]. Ann Oncol, 2019, 30(3): 385-396. doi: 10.1093/annonc/mdz003
[27] Billan S, Kaidar-Person O, Gil Z. Treatment after progression in the era of immunotherapy[J]. Lancet Oncol, 2020, 21(10): e463-e476. doi: 10.1016/S1470-2045(20)30328-4
[28] Mollaoglu G, Jones A, Wait SJ, et al. The lineage-defining transcription factors SOX2 and NKX2-1 determine lung cancer cell fate and shape the tumor immune microenvironment[J]. Immunity, 2018, 49(4): 764-779. e9. doi: 10.1016/j.immuni.2018.09.020
[29] Rosenthal R, Cadieux EL, Salgado R, et al. Neoantigen-directed immune escape in lung cancer evolution[J]. Nature, 2019, 567(7749): 479-485. doi: 10.1038/s41586-019-1032-7
[30] Xing XD, Yang F, Huang Q, et al. Decoding the multicellular ecosystem of lung adenocarcinoma manifested as pulmonary subsolid nodules by single-cell RNA sequencing[J]. Sci Adv, 2021, 7(5): eabd9738. doi: 10.1126/sciadv.abd9738
[31] Guo X, Zhang Y, Zheng L, et al. Publisher Correction: Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing[J]. Nat Med, 2018, 24(10): 1628.
[32] Ma KY, Schonnesen AA, Brock A, et al. Single-cell RNA sequencing of lung adenocarcinoma reveals heterogeneity of immune response-related genes[J]. JCI Insight, 2019, 4(4): e121387. doi: 10.1172/jci.insight.121387
[33] Zhang F, Bai H, Gao R, et al. Dynamics of peripheral T cell clones during PD-1 blockade in non-small cell lung cancer[J]. Cancer Immunol Immun, 2020, 69(12): 2599-2611.
[34] Chen Z, Yang X, Bi G, et al. Ligand-receptor interaction atlas within and between tumor cells and T cells in lung adenocarcinoma[J]. Int J Biol Sci, 2020, 16(12): 2205-2219. doi: 10.7150/ijbs.42080




 下载:
下载: