-
摘要:目的
探讨整合素β样1(ITGBL1)在肝细胞癌中的表达及其在促进肝癌细胞株增殖、迁移及侵袭中的作用。
方法实时荧光定量PCR和免疫组织化学法检测12例新鲜肝细胞癌和癌旁肝组织,以及160例石蜡样本中ITGBL1基因和蛋白的表达情况,并分析其表达水平与患者临床病理学参数及预后的关系;分别构建ITGBL1稳定过表达的HUH7细胞系及ITGBL1稳定下调表达的LM3细胞系,CCK8、划痕实验和Transwell实验检测ITGBL1对肝癌细胞株增殖、迁移和侵袭能力的影响。
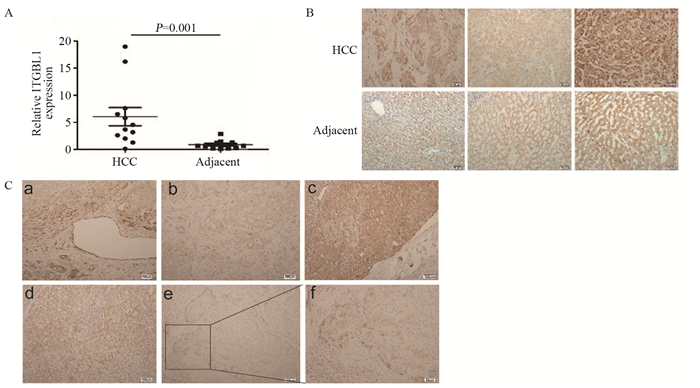
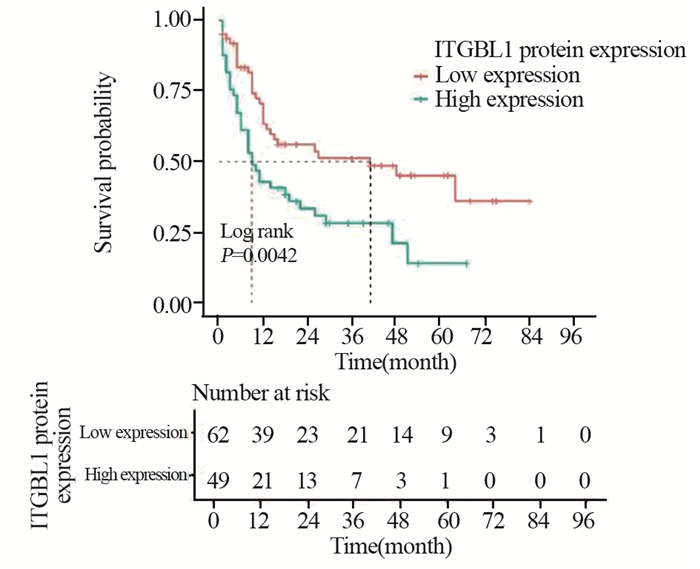
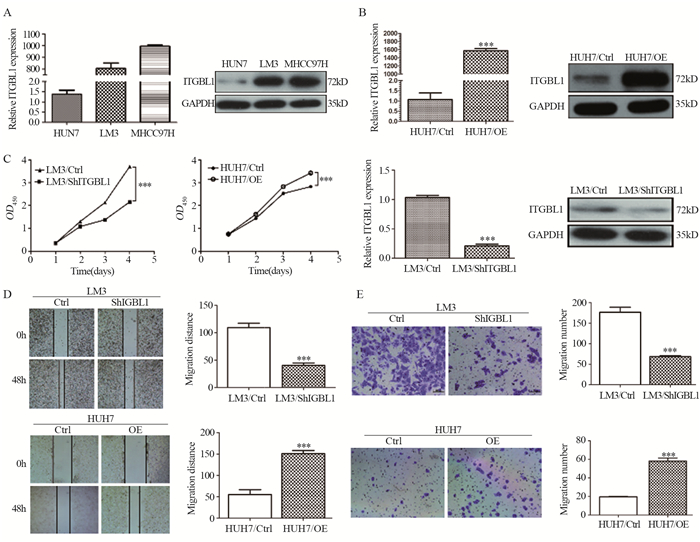
结果ITGBL1在癌组织中的表达高于癌旁肝组织,高表达ITGBL1与血清AFP水平、肿瘤包膜侵犯、脉管癌栓、肿瘤分化及临床分期相关(均P < 0.05),Kaplan-Meier单因素分析结果显示ITGBL1高表达患者DFS较短;过表达ITGBL1基因后,HUH7细胞的增殖、迁移及侵袭能力增强,而下调ITGBL1表达后LM3细胞的增殖、迁移及侵袭能力降低。
结论ITGBL1在肝细胞癌组织中高表达,ITGBL1可以促进肝癌细胞株的增殖、迁移及侵袭能力,但具体机制还需进一步研究。
Abstract:ObjectiveTo investigate the expression of ITGBL1 in hepatocellular carcinoma (HCC) and its role in promoting the proliferation, migration, and invasion of HCC cell lines.
MethodsRT-qPCR and immunohistochemistry were used in investigating ITGBL1 expression in 12 pairs of fresh HCC and adjacent normal liver tissue samples and 160 paraffin HCC specimens. The relationships of ITGBL1 expression level with clinicopathological parameters and prognosis were analyzed. HUH7 cell line with the stable overexpression of ITGBL1 and LM3 cell line with stable downregulated expression of ITGBL1 were constructed. The effects of ITGBL1 on the proliferation, migration, and invasion of HCC cells were examined by CCK8 assay, wound-healing assay, and Transwell invasion assay.
ResultsThe expression levels of ITGBL1 gene and protein in tumor tissues were higher than those in surrounding liver tissues. The high expression of ITGBL1 was correlated with serum AFP level, tumor capsular invasion, vascular invasion, tumor differentiation, and clinical stage (P < 0.05). The results of Kaplan-Meier analysis showed that patients with high expression of ITGBL1 had shorter DFS. In vitro, increased ITGBL1 expression promoted HCC cell proliferation, migration, and invasion, whereas ITGBL1 knockdown inhibited these processes.
ConclusionITGBL1 expression is highly expressed in HCC tissues, and ITGBL1 can increase the proliferation, migration, and invasion of HCC cells. However, the mechanism needs further study.
-
Key words:
- Hepatocellular carcinoma /
- Integrin subunit β-like 1 /
- Proliferation /
- Migration /
- Invasion
-
0 引言
反义寡核苷酸技术(antisense oligonucleotides, ASOs)又称反义技术,是指一种利用一段人工合成或表达的能与RNA或DNA互补结合的寡核苷酸链,抑制目的基因表达的技术[1-2],也可用于干扰肿瘤细胞中特定基因的表达,从而探讨肿瘤发生的机制和开发基因的治疗策略。近来研究显示,微小RNA-21(miR-21)与包括结直肠癌在内的多种肿瘤的发生密切相关[3-8],可能是肿瘤基因治疗的潜在新靶标[9-10]。我们在前期研究中发现,通过ASOs可显著下调人结肠癌SW620细胞中miR-21的表达,并抑制肿瘤的生长[11]。本研究中进一步利用人结肠癌HCT116细胞建立人结肠癌裸鼠实验动物模型,观察ASOs下调miR-21的效果及对人结肠癌细胞体内生长的效应,为开发基于ASOs技术的人结肠癌基因治疗策略提供实验基础。
1 材料与方法
1.1 实验动物、细胞株和主要试剂
12只雌性BALB/c裸鼠,4~5周龄,体质量11~14 g左右,购自北京维通利华实验动物技术有限公司[实验动物许可证编号:SCXK(京)2011-0011],于遵义医学院SPF级免疫学实验室人工饲养。人结肠癌HCT116细胞株购自中国生命科学院,重组质粒p-miR-21-ASOs和对照质粒p-Cont均由本实验室保存。McCoy's 5A培养基、磷酸盐缓冲液(PBS)均购自北京索莱宝科技有限公司,优质胎牛血清购自美国Gibco公司,100×三抗(青霉素-链霉素-两性霉素B)购自上海第四制药股份有限公司;SYBR Premix Ex Taq,Premix Ex Taq Version2.0以及RNAisoTM Plus均购自北京宝日医生物技术有限公司;miR-21探针试剂盒购自美国ABI公司;RevertAidTM First Strand cDNA Synthesis Kit反转录合成试剂盒购自美国Fermentas公司;总RNA提取试剂盒购自北京天根科技有限公司,0.5%Triton-X-100(聚乙二醇辛基苯基醚)购自北京索莱宝科技有限公司;APC标记的兔抗小鼠Ki-67单克隆抗体、DAPI染液均购自美国ThermoFisher公司;兔抗p-Akt、Akt一抗以及兔抗ERK1/2、p-ERK1/2一抗、HRP标记的羊抗兔二抗均购自英国Abcam公司;全蛋白提取试剂盒购自江苏凯基生物公司;ECL显色液、RIPA裂解液、PVDF膜购自中国联科生物技术公司。
1.2 方法
1.2.1 细胞培养
按如下条件培养人结肠癌HCT116细胞株:人结肠癌HCT116细胞株在10%优质胎牛血清、100 u/ml青霉素、100 μg/ml链霉素、0.25 μg/ml两性霉素B及11.9 g/L McCoy's 5A细胞培养液中培养,将人结肠癌HCT116细胞株置于37℃、5%CO2的条件下培养,待HCT116细胞处于对数生长期时,使用0.25%胰蛋白酶37℃消化2 min,离心收集细胞,使用PBS重复清洗3次,计算细胞总数,利用PBS调整HCT116细胞密度为4.5×107个/毫升备用。
1.2.2 建立人结肠癌裸鼠皮下移植瘤模型
4~5周龄裸鼠购回后先于本实验室SPF级环境下观察并饲养5~7 d。5~7 d天后小鼠饮食精神状况良好,随后接种100 μl密度为4.5×107个/毫升的人结肠癌HCT116细胞于裸鼠右侧腋窝外侧中部皮下,荷瘤后继续饲养并随时观察注射部位有无红肿溃烂、裸鼠的饮食、精神情况以及每只裸鼠荷瘤成功后的成瘤时间。接种后5~7 d出现肉眼可见的肿瘤包块时,即为荷瘤裸鼠模型构建成功。将实验荷瘤小鼠随机分为实验组(p-miR-21-ASOs组)和对照组(p-Cont组),每组6只,随后每隔4 d分别向两组荷瘤小鼠的肿瘤组织中注射100 μg p-Cont质粒和p-miR-21-ASOs质粒,共注射4次,并每隔3 d测量一次裸鼠皮下肿瘤体积。
1.2.3 观察荷瘤裸鼠体内肿瘤生长情况
观察方法同前期工作[11]。
1.2.4 免疫组织荧光法检测肿瘤组织中Ki-67蛋白的表达
从-80℃超低温冰箱取出用75%冰乙醇固定好的冰冻组织切片,室温(15℃~25℃)平衡10 min,PBS缓冲液漂洗3次,每次5 min;山羊血清封闭液封闭1.5 h,PBS漂洗3次,每次10 min;加0.5%Triton-X-100,冰上破膜2 min,PBS漂洗3次,每次10 min;吸弃封闭液,滴加APC标记的兔抗小鼠单克隆Ki-67抗体后置于湿盒内,4℃冰箱避光过夜孵育,次日,取出冰冻切片肿瘤组织用PBS缓冲液漂洗3次,每次10 min;滴加DAPI染液,染色3 min,PBS漂洗3次,每次10 min;滴加含有DAPI染液的封片剂,封片并固定24 h后观察;激光共聚焦显微镜观察细胞染色情况,拍片。
1.2.5 实时荧光定量PCR检测肿瘤组织中miR-21和肿瘤生长相关因子的表达
肿瘤组织中miR-21、细胞周期蛋白依赖性激酶(cyclin- dependent kinase, CDK)表达的检测同前期工作[11]。
1.2.6 Western blot检测Akt、ERK1/2、磷酸化Akt、磷酸化ERK1/2蛋白的表达
蛋白样品的制备及BCA法定量蛋白的检测同前期工作[11]。Western blot检测相关信号通路的变化:(1)电泳:分离胶10%,积层胶5%,80 V跑积层胶,120 V跑分离胶;(2)上样:第一泳道Marker,其余泳道加提取的总蛋白;(3)转膜:PVD膜,36 V,180 min电转;(4)洗膜:用TBS或者PBS洗膜;5 min一次;(5)封闭:用含5%脱脂奶粉的TBST或者PBST封闭1 h;(6)加一抗:加入相关目的蛋白的一抗(稀释比例均为1:2 000),4℃过夜;(7)洗膜:次日用TBST或者PBST洗膜,10 min三次;(8)加二抗:加入HRP标记的相关目的蛋白的二抗(稀释比例均为1:2 000),室温孵育1 h;(9)用PBST洗膜,10 min×3次;(10)用发光试剂(ECl)对膜进行处理,于发光仪上进行蛋白曝光检测。
1.3 统计学方法
实验数据使用GraphPad Prism 5软件进行统计学分析。本研究中各实验数据均独立重复3次,结果以(x±s)表示。其中组间比较采用t检验或单因素方差分析,P < 0.05为差异有统计学意义。
2 结果
2.1 p-miR-21-ASOs重组质粒注射后各组裸鼠荷瘤模型中肿瘤的生长变化
结果显示,与p-Cont组相比,p-miR-21-ASOs组皮下肿瘤生长显著延缓,且肿瘤组织的体积、大小、重量都明显减小,差异有统计学意义,见图 1。
2.2 肿瘤组织病理学改变
HE染色显示:p-Cont组肿瘤细胞形态不一,细胞核深染且核呈多形性,核质比加大;而p-miR-21-ASOs组则可见肿瘤组织大面积坏死,见图 2。
2.3 p-miR-21-ASOs重组质粒注射后肿瘤组织中miR-21成熟体的表达
结果显示:与p-Cont组相比,p-miR-21-ASOs组肿瘤组织中miR-21的表达水平显著下调,且差异有统计学意义(P < 0.05),见图 3。
2.4 免疫荧光技术检测肿瘤组织中细胞的增殖
免疫荧光共聚焦观察发现:相比于p-Cont组,p-miR-21-ASOs组肿瘤组织中Ki-67的表达水平明显减少(P < 0.01),见图 4。
2.5 p-miR-21-ASOs重组质粒注射后周期蛋白依赖性激酶的表达
结果显示:与p-Cont组相比,p-miR-21-ASOs组肿瘤细胞生长周期相关的周期蛋白依赖性激酶CDK2、CDK3、CDK4以及CDK6的表达水平均明显下调,差异有统计学意义(P < 0.05, P < 0.001),见图 5。
2.6 Western blot检测肿瘤组织中相关信号通路的表达
结果显示:与p-Cont组相比,p-miR-21-ASOs组中p-ERK1/2,p-Akt的蛋白表达水平明显降低(P < 0.05, P < 0.01),见图 6。
3 讨论
近来的研究发现,ASOs技术可广泛用于多种临床疾病发生机制探讨和治疗策略开发[12-18]。对于肿瘤基因治疗来说,利用ASOs技术干预特定基因表达来影响肿瘤生长方面的研究也取得了重要进展[19-20]。我们在新近工作中也发现利用真核载体过表达ASOs干预miR-21表达可显著抑制人结肠癌SW620细胞的体内生长[11]。本研究中,我们进一步发现,真核载体过表达ASOs也可有效抑制人结肠癌HCT116细胞中miR-21的表达,同时肿瘤细胞体内生长受到抑制。范钰等[21]用ASOs下调人喉癌Hep-2细胞中miR-21的表达,发现肿瘤细胞的生长、侵袭能力明显减弱。吴子晏等[22]研究发现ASOs干预miR-21联合顺铂在体内、外能显著抑制人骨肉瘤MG-63细胞生长,并促进细胞凋亡,显示了一定的治疗效应。这些研究结果提示,利用ASOs技术干预miR-21表达可能是一种能有效影响特定肿瘤生长的手段,为后续开发基于ASOs的人结肠癌基因治疗策略提供了重要工作基础。
研究显示,miR-21可通过对多种靶分子和信号途径传递的调节来调控人结肠癌的生长[23-28]。在前期工作中,我们也发现ASOs下调miR-21表达后,肿瘤细胞生长相关信号途径Akt和ERK1/2明显改变,与其靶分子PTEN的表达变化有关[4, 10]。本研究进一步发现ASOs下调miR-21表达后,肿瘤组织存在坏死,肿瘤细胞的增殖能力明显减弱,同时,肿瘤组织中p-Akt和p-ERK1/2等蛋白的表达水平均明显下调,进一步提示了miR-21对Akt和ERK1/2等信号途径的调控作用。现已知,细胞内CDKs家族分子的表达改变与肿瘤细胞生长相关。本研究也发现肿瘤细胞内CDKs家族成员表达水平显著减低。鉴于已有的研究表明,肿瘤细胞生长相关信号途径Akt和ERK1/2与细胞内CDKs家族成员表达间存在密切联系[29-31]。因此,推测ASOs干预miR-21表达后对人结肠癌细胞体内生长的抑制效应机制,包括肿瘤组织坏死的变化,与Akt和ERK1/2等信号途径传递改变,进而影响CDKs家族分子的表达有关。然而,鉴于不同实验条件的差异性,ASOs下调miR-21后抑制人结肠癌细胞体内生长的确切分子机制仍有待后续研究阐明。
总之,本研究发现ASOs可显著下调人结肠癌动物模型中肿瘤组织miR-21的表达,同时肿瘤体内生长受到明显抑制,该效应与Akt和ERK1/2等信号途径传递变化有关,为后续深入探讨miR-21调控肿瘤生长的分子机制,以及开发基于ASOs技术的人结肠癌基因治疗策略提供前期实验基础。
Competing interests: The authors declare that they have no competing interests.作者贡献:林丽燕:实验设计与操作、文章撰写卢建平、何时:免疫组织化学结果判读与临床资料收集朱伟峰:病理切片的制备彭凤英:免疫组织化学染色陈刚:实验设计与文章修改 -
表 1 160例肝细胞癌患者的一般临床资料
Table 1 Clinical features of 160 patients with hepatocellular carcinoma

表 2 ITGBL1蛋白表达水平与临床病理特征的关系(N=160)
Table 2 Correlation between ITGBL1 protein expression and clinicopathological features in patients with HCC (N=160)

表 3 单因素Log rank分析可能影响HCC患者术后DFS的预后因素
Table 3 Univariate Log rank analysis to identify potential prognostic factors affecting DFS of HCC patients

表 4 多因素Cox回归分析影响HCC患者术后DFS的独立预后因素
Table 4 Multivariate Cox regression analysis to identify independent prognostic factors affecting DFS in HCC patients

-
[1] Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. doi: 10.3322/caac.21660
[2] Cao W, Chen HD, Yu YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020[J]. Chin Med J (Engl), 2021, 134(7): 783-791. doi: 10.1097/CM9.0000000000001474
[3] Berg RW, Leung E, Gough S, et al. Cloning and Characterization of a Novel β Integrin-Related cDNA Coding for the Protein TIED ("Ten β Integrin EGF-like Repeat Domains") That Maps to Chromosome Band 13q33: A Divergent Stand-Alone Integrin Stalk Structure[J]. Genomics, 1999, 56(2): 169-178. doi: 10.1006/geno.1998.5707
[4] Li R, Zhuang C, Jiang S, et al. ITGBL1 Predicts a Poor Prognosis and Correlates EMT Phenotype in Gastric Cancer[J]. J Cancer, 2017, 8(18): 3764-3773. doi: 10.7150/jca.20900
[5] Shan M, Xia Q, Yan D, et al. Molecular analyses of prostate tumors for diagnosis of malignancy on fine-needle aspiration biopsies[J]. Oncotarget, 2017, 8(62): 104761-104771. doi: 10.18632/oncotarget.22289
[6] Matsuyama T, Ishikawa T, Takahashi N, et al. Transcriptomic expression profiling identifies ITGBL1, an epithelial to mesenchymal transition (EMT)-associated gene, is a promising recurrence prediction biomarker in colorectal cancer[J]. Mol Cancer, 2019, 18(1): 19. doi: 10.1186/s12943-019-0945-y
[7] Song J, Yang P, Lu J. Upregulation of ITGBL1 predicts poor prognosis and promotes chemoresistance in ovarian cancer[J]. Cancer Biomark, 2020, 27(1): 51-61.
[8] Baglieri J, Brenner DA, Kisseleva T. The Role of Fibrosis and Liver-Associated Fibroblasts in the Pathogenesis of Hepatocellular Carcinoma[J]. Int J Mol Sci, 2019, 20(7): 1723. doi: 10.3390/ijms20071723
[9] Xu MY, Qu Y, Li Z, et al. A 6 gene signature identifies the risk of developing cirrhosis in patients with chronic hepatitis B[J]. Front Biosci (Landmark Ed), 2016, 21(3): 479-486. doi: 10.2741/4403
[10] Wang M, Gong Q, Zhang J, et al. Characterization of gene expression profiles in HBV-related liver fibrosis patients and identification of ITGBL1 as a key regulator of fibrogenesis[J]. Sci Rep, 2017, 7: 43446. doi: 10.1038/srep43446
[11] Wang Z, Fu L, Zhang J, et al. A comprehensive analysis of potential gastric cancer prognostic biomarker ITGBL1 associated with immune infiltration and epithelial-mesenchymal transition[J]. Biomed Eng Online, 2022, 21(1): 30. doi: 10.1186/s12938-022-00998-5
[12] Qiu X, Feng JR, Qiu J, et al. ITGBL1 promotes migration, invasion and predicts a poor prognosis in colorectal cancer[J]. Biomed Pharmacother, 2018, 104: 172-180. doi: 10.1016/j.biopha.2018.05.033
[13] 中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗指南(2022年版)[J]. 中华消化外科杂志, 2022, 21(2): 143-168. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGWK202203001.htm Medical Administration Bureau of the National Health Commission of the People's Republic of China. Standardization for diagnosis and treatment of hepatocellular carcinoma (2022 edition)[J]. Zhonghua Xiao Hua Wai Ke Za Zhi, 2022, 21(2): 143-168. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGWK202203001.htm
[14] Qi L, Song F, Ding Y. Regulatory Mechanism of ITGBL1 in the Metastasis of Colorectal Cancer[J]. Front Oncol, 2020, 10: 259. doi: 10.3389/fonc.2020.00259
[15] Yin FY, Qi HP, Qiao H, et al. ITGBL1 promotes gastric cancer cell proliferation and invasion via Akt signal pathway[J]. Front Biosci (Landmark Ed), 2021, 26(4): 682-691. doi: 10.2741/4912
[16] Li W, Li S, Yang J, et al. ITGBL1 promotes EMT, invasion and migration by activating NF-κB signaling pathway in prostate cancer[J]. Onco Targets Ther, 2019, 12: 3753-3763. doi: 10.2147/OTT.S200082
[17] Huang W, Yu D, Wang M, et al. ITGBL1 promotes cell migration and invasion through stimulating the TGF-β signalling pathway in hepatocellular carcinoma[J]. Cell Prolif, 2020, 53(7): e12836.
[18] Northey JJ, Przybyla L, Weaver V. Tissue Force Programs Cell Fate and Tumor Aggression[J]. Cancer Discov, 2017, 7(11): 1224-1237. doi: 10.1158/2159-8290.CD-16-0733
[19] Ji Q, Zhou L, Sui H, et al. Primary tumors release ITGBL1-rich extracellular vesicles to promote distal metastatic tumor growth through fibroblast-niche formation[J]. Nat Commun, 2020, 11(1): 1211. doi: 10.1038/s41467-020-14869-x
[20] Li XQ, Du X, Li DM, et al. ITGBL1 Is a Runx2 Transcriptional Target and Promotes Breast Cancer Bone Metastasis by Activating the TGF-β Signaling Pathway[J]. Cancer Res, 2015, 75(16): 3302-3313. doi: 10.1158/0008-5472.CAN-15-0240
[21] Ye F, Huang W, Xue Y, et al. Serum Levels of ITGBL1 as an Early Diagnostic Biomarker for Hepatocellular Carcinoma with Hepatitis B Virus Infection[J]. J Hepatocell Carcinoma, 2021, 8: 285-300. doi: 10.2147/JHC.S306966
[22] Cheli Y, Tulic MK, El Hachem N, et al. ITGBL1 is a new immunomodulator that favors development of melanoma tumors by inhibiting natural killer cells cytotoxicity[J]. Mol Cancer, 2021, 20(1): 12. doi: 10.1186/s12943-020-01306-2




 下载:
下载: