Clinical Value of Platelet and Its Parameters Combined with Tumor Markers in Preoperative Differentiation of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma
-
摘要:目的
探讨血小板(PLT)及其参数和甲胎蛋白(AFP)、糖类抗原199(CA199)、糖类抗原125(CA125)及癌胚抗原(CEA)在术前肝细胞癌(HCC)及肝内胆管癌(ICC)鉴别诊断中的价值。
方法回顾性分析兰州大学第二医院行手术治疗的肝癌患者274例,据术后病理将其分为HCC组229例和ICC组45例。比较两组PLT及其参数和肿瘤标志物水平的差异;受试者工作特征(ROC)曲线评价有显著差异的指标进行单独和联合时对HCC和ICC的鉴别诊断效能,并将最佳方案应用于术前明确诊断和非明确诊断患者中进行验证。
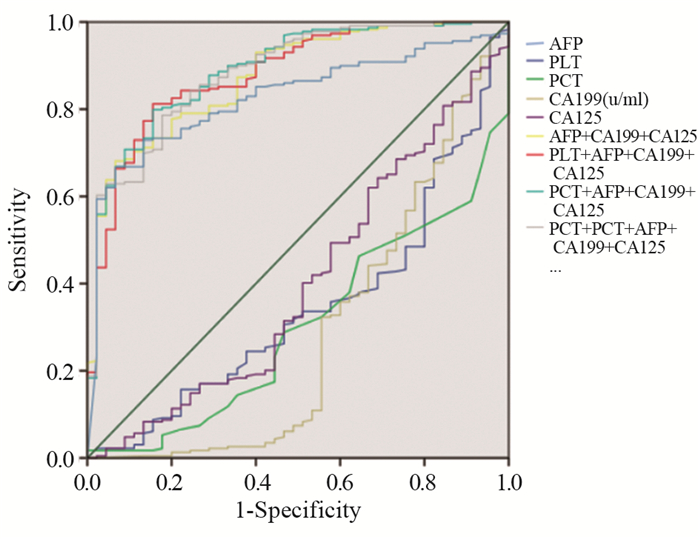
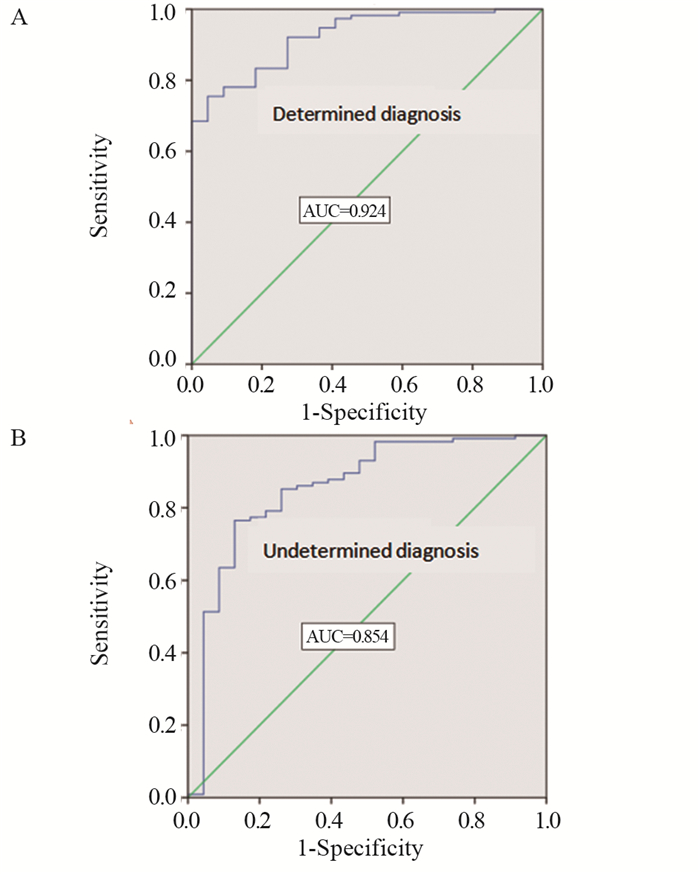
结果与HCC组相比,ICC组的PLT、血小板压积(PCT)、CA199和CA125水平较高(P < 0.05),AFP水平较低(P < 0.05),其余指标差异无统计学意义(P > 0.05)。ROC曲线诊断分析结果显示:单独检测时,AFP对HCC诊断的曲线下面积(AUC)最大,为0.827。肿瘤标志物的联合方案均较单独方案的AUC大,其中以PCT+AFP+CA199+CA125联合方案鉴别诊断效能最佳,AUC为0.891。对最佳联合方案验证表明,其在术前明确诊断组的AUC为0.924,在非明确诊断组的AUC为0.854。
结论联合PLT、PCT可以增加现有肿瘤标志物单独对术前HCC和ICC的鉴别诊断效能。术前行PCT+AFP+CA199+CA125联合方案有助于进一步明确诊断和规划手术方案。
Abstract:ObjectiveTo evaluate the value of PLT and its parameters combined with AFP, CA199, CA125 and CEA on the preoperative differential diagnosis of hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC).
MethodsWe analyzed retrospectively 274 patients with liver cancer who underwent surgery in the Second Hospital of Lanzhou University. They were divided into 229 cases in HCC group and 45 cases in ICC group according to postoperative pathological results. The differences of PLT, its parameters and tumor markers between the two groups were compared. ROC curve was used to evaluate the differential diagnosis effect on HCC and ICC by significantly different indicators in single and combined forms. The best scheme was verified in the patients with determined and undetermined preoperative diagnosis.
ResultsCompared with HCC group, the levels of PLT, PCT, CA199 and CA125 in ICC group were higher (P < 0.05) and the level of AFP was lower (P < 0.05). The diagnostic analysis results of ROC curve showed that in single test, the AUC of AFP for HCC diagnosis was the largest (0.827). The AUC of the combined groups was higher than the single groups of tumor markers; the AUC of the PCT+AFP+CA199+CA125 group was the highest in all combination groups, and AUC was 0.891. The verification of the best combination group showed that the AUC was 0.924 in the preoperative determined diagnosis group and 0.854 in the undetermined diagnosis group.
ConclusionTumor markers in combination with PLT and PCT can increase the preoperative differential diagnosis efficacy of HCC and ICC. The combination of PCT, AFP, CA199 and CA125 before operation is helpful to further determine the diagnosis and plan the operation scheme.
-
Key words:
- Hepatocellular carcinoma /
- Intrahepatic cholangiocarcinoma /
- Platelet /
- AFP /
- CA199 /
- CA125 /
- CEA
-
0 引言
鼻咽癌是最常见的头颈部肿瘤之一。我国为鼻咽癌高发地区,每年的发病率约为20/10万[1],由于鼻咽解剖结构及生物学行为的特殊性,很难行手术治疗,目前鼻咽癌公认和有效的治疗手段为放射治疗或以放疗为主的综合治疗。虽然放疗技术不断进步与放疗设备的不断更新,鼻咽癌的生存率有了较大的提高,但5年生存率仍为60%~80%[2],部分患者仍未能获得长期生存。TNM分期系统是鼻咽癌预后判断和指导治疗的重要依据,但临床发现同一分期患者即使接受相同的治疗方案,预后却不同[3-4],这提示鼻咽癌生物学差异的存在,仅基于解剖学信息的TNM临床分期系统还不能准确地预测鼻咽癌患者的预后。虽然EB病毒滴度、表皮生长因子受体、microRNA也可提示鼻咽癌的预后[5-7],但检测成本高,需要多中心合作,临床上可行性差。所以,亟需检测方便、价格低廉可预测鼻咽癌预后的标志物。
流行病学研究证实,约25%的肿瘤由炎性反应发展而来,其与肿瘤的发生发展密切相关并且影响肿瘤患者的预后[8]。炎性反应指标,如白细胞计数[9]、血小板计数[10-11]、中性粒淋巴细胞比(neutrophil-lymphocyte ratio, NLR)[12-13]、血小板淋巴细胞比(platelet-lymphocyte ratio, PLR)[14-15]被发现可作为肿瘤的独立预后因素。这些血液指标检测方便,价格低廉,可广泛应用于临床,评估患者预后。本研究通过对91例鼻咽癌患者临床资料进行回顾性分析,评价治疗前PLR和NLR与鼻咽癌患者预后的相关性,为评估预后提供客观依据。
1 资料与方法
1.1 临床资料
回顾性收集2009年1月至2013年9月期间于西安交通大学第一附属医院和陕西省人民医院初治的91例鼻咽癌患者,所有病例均经病理证实。临床资料完整。排除标准:(1)合并有免疫性疾病以及其他恶性肿瘤的患者;(2)治疗前合并有急性或慢性感染;(3)合并有血液系统疾病、血栓或出血性疾病;(4)合并有严重的肝、肾疾病;(5)治疗前曾接受过放疗或化疗;(6)无远处转移。记录患者治疗前的中性粒细胞计数、淋巴细胞计数及血小板计数结果。
1.2 治疗及随访方法
入选患者采用3D-CRT或IMRT根治性放疗(有或无化疗),Ⅰ期患者仅接受单纯放射治疗,Ⅱ、Ⅲ、Ⅳ期患者接受以顺铂和5-氟尿嘧啶为主的辅助或同步放化疗。鼻咽原发灶和颈部转移淋巴结剂量为(70~76)Gy/(7~8)w/(35~38)f,颈部预防区域剂量为(50~60)Gy/(5~6)w/(25~30)f。根据患者的临床分期及不良反应给予2~6周期的全身化疗,化疗方案为:顺铂25 mg/m2,第1~3天静脉滴注;5-氟尿嘧啶500 mg/m2,第1~5天静脉滴注,每21天重复1周期。患者治疗结束后均定期随访,治疗后前2年,每3月检查一次,2年后半年复查一次,5年后1年复查1次。随访截止时间为2016年9月。
1.3 统计学方法
采用SPSS19.0软件对数据进行统计学分析。绘制ROC曲线确定PLR和NLR与总生存期(overall survival, OS)及无进展生存期(progression-free survival, PFS)的相关性,选取截断值。应用Kaplan-Meier法进行生存分析并采用Log rank检验来检验。采用Cox比例风险回归模型分析多种因素对生存时间的影响。以P < 0.05为差异有统计学意义。
2 结果
2.1 鼻咽癌患者临床病理资料
91例患者的基本特征资料见表 1,中位年龄53岁(12~72)岁,女30例,男61例,男女比例2:1,Ⅰ、Ⅱ、Ⅲ、Ⅳ期患者分别为2、27、42、20例。单纯放疗患者9例,82例患者接受辅助或同步放化疗,所有患者均按期完成放化疗。中位随访时间为44月(6~87)月,其中44例出现复发或转移,39例患者死亡。患者的1、3、5年总生存率分别为92.3%、72.1%、56.8%,1、3、5年无进展生存率分别为82.4%、60.9%、53.3%。
表 1 91例鼻咽癌患者临床基本特征资料(n(%))Table 1 Basic clinical features of 91 nasopharyngeal carcinoma patients (n(%))
2.2 ROC曲线选取PLR和NLR预后相关截断值
以OS作为终点,PLR、NLR为检测变量,绘制ROC曲线选取截断值分别为143.3、2.6,两者的曲线下面积分别为0.640、0.739,见图 1。
以PFS作为终点,PLR、NLR为检测变量,绘制ROC曲线选取截断值分别为143.3、2.6,两者的曲线下面积分别为0.657、0.694,见图 2。说明治疗前PLR、NLR与患者的预后存在相关性,利用ROC曲线选取的截断值进行进一步生存分析。
2.3 Kaplan-Meier生存分析、Cox单因素和多因素分析
PLR≥143.3组和PLR < 143.3组患者生存曲线比较,差异有统计学意义(P=0.022),见图 3~4。NLR≥2.6组和NLR < 2.6组患者生存曲线比较,差异有统计学意义(P=0.044),见图 5~6。
Cox单因素分析显示除性别、年龄以外,TNM分期、治疗前PLR≥143.3、NLR≥2.6均是影响鼻咽癌患者OS和PFS的不良预后因素(P < 0.05),见表 2。Cox多因素分析显示治疗前PLR≥143.3(RR=2.491, 95%CI=1.139~5.451, P=0.022)、NLR≥2.6(RR=2.186, 95%CI=1.021~4.682,P=0.044)是鼻咽癌患者OS的独立危险因素,而治疗前PLR≥143.3(RR=2.461,95%CI=1.242~4.874, P=0.010)是鼻咽癌患者PFS的独立危险因素,见表 3。
表 2 影响鼻咽癌患者生存预后的Cox单因素分析Table 2 Cox univariate analysis of prognostic factors for nasopharyngeal carcinoma patients 表 3 影响鼻咽癌患者生存预后的Cox多因素分析Table 3 Cox multivariate analysis of prognostic factors for nasopharyngeal carcinoma patients
表 3 影响鼻咽癌患者生存预后的Cox多因素分析Table 3 Cox multivariate analysis of prognostic factors for nasopharyngeal carcinoma patients
3 讨论
鼻咽癌对放射线高度敏感,因此放疗成为主要治疗手段。随着三维适形放疗和调强放射治疗的临床应用,鼻咽癌的生存率较前明显提高,但5年生存率仍仅为60%~80%。多项研究表明鼻咽癌患者预后与众多因素有关,包括患者年龄、临床分期、EB病毒感染及贫血等。此外,肿瘤的预后还与机体本身的炎性反应有关。炎性反应包含中性粒细胞、淋巴细胞、血小板、C反应蛋白等多种指标,其中PLR、NLR已受到越来越多专家的关注。本研究发现治疗前PLR和NLR可能成为鼻咽癌的独立预后因素。
恶性肿瘤患者常伴随血小板的升高,实验研究表明血小板参与肿瘤细胞生长、转移及血管生成[16]。临床研究表明血小板数目升高与肿瘤患者较差预后相关[11, 17]。此外研究表明中性粒细胞可促使机体产生多种促肿瘤生长因子和蛋白酶,促进肿瘤的发生、发展[18]。而淋巴细胞参与机体的免疫反应是抗肿瘤免疫的重要组成部分,淋巴细胞减少说明机体免疫机制异常,抗肿瘤免疫力下降,为肿瘤生长、浸润和转移提供条件。随着肿瘤进展,机体内炎性反应与肿瘤失去平衡,体内淋巴细胞降低,而血小板、中性粒细胞升高,相应的PLR和NLR比值也增高,机体内促进肿瘤炎性反应与抗肿瘤炎性反应的平衡状态被打破。因此PLR和NLR是反应机体免疫情况的重要指标,两者的升高能促进肿瘤进展,导致肿瘤患者预后不良。既往研究结果显示高PLR和NLR可影响宫颈癌、乳腺癌、结直肠癌等恶性肿瘤的预后[19-21]。而目前关于PLR、NLR与鼻咽癌患者预后相关性的研究较少,Sun等[21]分析了251例鼻咽癌患者治疗前PLR和NLR,结果证明治疗前两者水平是影响鼻咽癌患者生存独立预后因素。本研究结果显示治疗前PLR、NLR与鼻咽癌患者的总生存期和无进展生存期具有相关性。Cox多因素分析提示PLR≥143.3、NLR≥2.6和TNM分期是影响鼻咽癌患者治疗后的独立危险因素。PLR≥143.3组患者有较短OS和PFS,而NLR≥2.6组患者有较差的OS,和本研究结果相一致。因此,高PLR、NLR的鼻咽癌患者总生存率要低于低PLR、NLR的患者,且高PLR的患者复发或转移风险明显增加。据此,临床上或许可以通过提高鼻咽癌患者免疫功能及降低机体炎性反应,改善患者的预后。
但由于本研究是一个相对小样本的回顾性研究,不能代表大部分的鼻咽癌患者,且随访时间较短,存在一定的局限性,因此需要进行多中心、大样本的前瞻性研究来进一步证实。
本研究结果表明,治疗前PLR和NLR水平与鼻咽癌患者预后具有相关性,可能是影响鼻咽癌患者预后的独立危险因素,NLR和PLR的获取具有简便、经济的优点,可以作为鼻咽癌患者病情评估的一个有益补充,值得推广。目前鼻咽癌相关有效预后指标较多,笔者将在今后的临床研究工作中继续探索,将本研究指标与已有的有效预后指标进行比较,从而提高治疗前PLR和NLR水平这一预后指标应用于临床的合理性及可靠性。
Competing interests: The authors declare that they have no competing interests.作者贡献:张浩东:数据收集和统计分析,构思和撰写文章魏丰贤:指导文章撰写张春芳:文章内容修改润色徐小东:指导文章整体构思和内容修改 -
表 1 肝细胞癌(HCC)组和肝内胆管癌(ICC)组的基线特征
Table 1 Baseline characteristics of HCC and ICC groups

表 2 HCC组和ICC组的PLT及其参数、肿瘤标志物比较
Table 2 Comparison of PLT, its parameters and tumor markers between HCC and ICC groups

表 3 PLT、PCT、CA199及CA125对ICC预测的ROC曲线分析结果
Table 3 ROC curve analysis of ICC predicted by PLT, PCT, CA199 and CA125

表 4 AFP单独以及与CA199、CA125、PLT、PCT联合对HCC预测的ROC曲线分析结果
Table 4 ROC curves analysis of HCC predicted by combination of PLT, PCT, AFP, CA199 and CA125 as well as single AFP

-
[1] Sia D, Villanueva A, Friedman SL, et al. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis[J]. Gastroenterology, 2017, 152(4): 745-761. doi: 10.1053/j.gastro.2016.11.048
[2] Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma[J]. Cancer Control, 2017, 24(3): 1073274817729245.
[3] Brown KM, Parmar AD, Geller DA. Intrahepatic cholangiocarcinoma[J]. Surg Oncol Clin N Am, 2014, 23(2): 231-246. doi: 10.1016/j.soc.2013.10.004
[4] 中华人民共和国国家卫生健康委员会医政医管局. 原发性肝癌诊疗规范(2019年版)[J]. 中华肝脏病杂志, 2020, 28(2): 112-128. doi: 10.3760/cma.j.issn.1007-3418.2020.02.004 Department of Medical Administration, National Health and Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)[J]. Zhonghua Gan Zang Bing Za Zhi, 2020, 28(2): 112-128. doi: 10.3760/cma.j.issn.1007-3418.2020.02.004
[5] 杭轶, 杨小勇, 李文美. 肝内胆管癌与肝细胞癌临床特征的比较研究[J]. 中国普通外科杂志, 2015, 24(2): 175-179. https://www.cnki.com.cn/Article/CJFDTOTAL-ZPWZ201502007.htm Hang Y, Yang XY, Li WM. Comparative study of clinical features between intrahepatic cholangiocarcinoma and hepatocellular carcinoma[J]. Zhongguo Pu Tong Wai Ke Za Zhi, 2015, 24(2): 175-179. https://www.cnki.com.cn/Article/CJFDTOTAL-ZPWZ201502007.htm
[6] 陈丽华, 刘爱连, 宋清伟, 等. 磁共振扩散张量成像鉴别诊断肝内胆管细胞癌与肝细胞癌[J]. 中国医学影像技术, 2017, 33(7): 993-997. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXX201707011.htm Chen LH, Liu AL, Song QW, et al. Diffusion tensor imaging in differential diagnosis of intrahepatic cholangiocarcinoma and hepatocellular carcinoma[J]. Zhongguo Yi Xue Ying Xiang Ji Shu, 2017, 33(7): 993-997. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXX201707011.htm
[7] Wang K, Zhang H, Xia Y, Liu J, et al. Surgical options for intrahepatic cholangiocarcinoma[J]. Hepatobiliary Surg Nutr, 2017, 6(2): 79-90. doi: 10.21037/hbsn.2017.01.06
[8] Macias RIR, Kornek M, Rodrigues PM, et al. Diagnostic and prognostic biomarkers in cholangiocarcinoma[J]. Liver Int, 2019, 39 Suppl 1: 108-122.
[9] Banales JM, Iñarrairaegui M, Arbelaiz A, et al. Serum Metabolites as Diagnostic Biomarkers for Cholangiocarcinoma, Hepatocellular Carcinoma, and Primary Sclerosing Cholangitis[J]. Hepatology, 2019, 70(2): 547-562. doi: 10.1002/hep.30319
[10] Blechacz B, Komuta M, Roskams T, et al. Clinical diagnosis and staging of cholangiocarcinoma[J]. Nat Rev Gastroenterol Hepatol, 2011, 8(9): 512-522. doi: 10.1038/nrgastro.2011.131
[11] 王冲, 程石. 肝内胆管癌—国内外专家共识及指南解读[J]. 外科理论与实践, 2021, 26(2): 124-129. https://www.cnki.com.cn/Article/CJFDTOTAL-WKLL202102012.htm Wang C, Cheng S. Intrahepatic cholangiocarcinoma- consensus and guideline interpretation of experts at home and abroad[J]. Wai Ke Li Lun Yu Shi Jian, 2021, 26(2): 124-129. https://www.cnki.com.cn/Article/CJFDTOTAL-WKLL202102012.htm
[12] Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012[J]. CA Cancer J Clin, 2015, 65(2): 87-108. doi: 10.3322/caac.21262
[13] Zhang H, Yang T, Wu M, et al. Intrahepatic cholangiocarcinoma: Epidemiology, risk factors, diagnosis and surgical management[J]. Cancer Lett, 2016, 379(2): 198-205. doi: 10.1016/j.canlet.2015.09.008
[14] 安澜, 冉显会, 郑荣寿, 等. 中国肝细胞癌和肝内胆管细胞癌临床诊疗情况比较研究[J]. 中国癌症防治杂志, 2021, 13(2): 126-132. doi: 10.3969/j.issn.1674-5671.2021.02.03 An L, Ran XH, Zheng RS, et al. A comparative study on clinical diagnosis and treatment of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in China[J]. Zhongguo Ai Zheng Fang Zhi Za Zhi, 2021, 13(2): 126-132. doi: 10.3969/j.issn.1674-5671.2021.02.03
[15] Chan TS, Hwang YY, Gill HS, et al. Increasing incidence of venous thromboembolism due to cancer-associated thrombosis in Hong Kong Chinese[J]. Throm Res, 2014, 134(5): 1157-1159. doi: 10.1016/j.thromres.2014.08.008
[16] Walker AJ, Grainge MJ, Card TR, et al. Venous thromboembolism in children with cancer-A population-based cohort study[J]. Throm Res, 2014, 133(3): 340-344. doi: 10.1016/j.thromres.2013.12.021
[17] Nailin Li. Platelets in cancer metastasis: To help the "villain" to do evil[J]. Int J Cancer, 2016, 138(9): 2078-2087. doi: 10.1002/ijc.29847
[18] Best MG, Sol N, Kooi I, et al. RNA-Seq of tumor-educated platelets enables blood-based pan-cancer, multiclass, and molecular pathway cancer diagnostics[J]. Cancer Cell, 2015, 28(5): 666-676. doi: 10.1016/j.ccell.2015.09.018
[19] Nilsson RJ, Balaj L, Hulleman E, et al. Blood platelets contain tumor-derived RNA biomarkers[J]. Blood, 2011, 118(13): 3680-3683. doi: 10.1182/blood-2011-03-344408
[20] Cetin M, Bakirci EM, Baysal E, et al. Increased platelet distribution width is associated with ST-segment elevation myocardial infarction and thrombolysis failure[J]. Angiology, 2014, 65(8): 737-743. doi: 10.1177/0003319713520068
[21] Yan M, Lesyk G, Radziwon-Balicka A, et al. Pharmacological Regulation of Platelet Factors That Influence Tumor Angiogenesis[J]. Semin Oncol, 2014, 41(3): 370-377. doi: 10.1053/j.seminoncol.2014.04.007
[22] Szubert S, Moszynski R, Szpurek D, et al. The expression of Platelet-derived Growth factor receptors (PDGFRs) and their correlation with overall survival of patients with ovarian cancer[J]. Ginekol Pol, 2019, 90(5): 242-249. doi: 10.5603/GP.a2019.0045
[23] D'Oronzo S, Brown J, Coleman R. The role of biomarkers in the management of bone-homing malignancies[J]. J Bone Oncol, 2017, 9: 1-9. doi: 10.1016/j.jbo.2017.09.001
[24] 董超男, 翟文萍, 王雪野. 血小板介导肿瘤细胞生长和转移的机制研究进展[J]. 医学综述, 2020, 26(4): 695-699. doi: 10.3969/j.issn.1006-2084.2020.04.014 Dong CN, Zhai WP, Wang XY. Research progress in mechanism of platelet-mediated tumor cell growth and metastasis[J]. Yi Xue Zong Shu, 2020, 26(4): 695-699. doi: 10.3969/j.issn.1006-2084.2020.04.014



 下载:
下载: