Efficacy and Safety of Sacituzumab Govitecan on Advanced HER2-negative Breast Cancer: A Meta-analysis
-
摘要:目的
探究Sacituzumab govitecan对晚期HER2阴性乳腺癌患者的疗效及安全性。
方法分类整理截至2021年5月发表的相关的Ⅱ期或Ⅲ期临床试验,并使用Stata12.0软件包分析Sacituzumab govitecan在晚期HER2阴性乳腺癌患者中的疗效和不良反应。
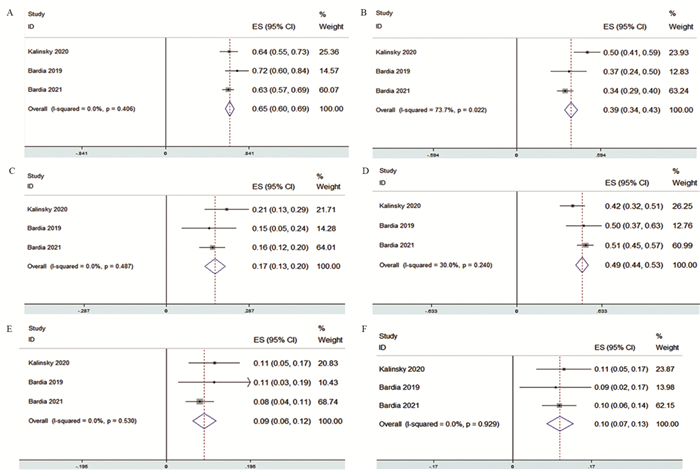
结果最终纳入3篇文献,420例晚期HER2阴性乳腺癌患者。其中随机对照试验研究1篇,队列研究2篇。Meta分析结果显示:使用Sacituzumab govitecan的患者,缓解率为34.0%(95%CI: 29.4%~38.6%)、临床获益率为45.0%(95%CI: 40.2%~49.8%)、中位无进展生存为5.59月(95%CI: 4.95~6.31)、中位总生存为12.59月(95%CI: 11.58~13.68)、中位反应持续时间为7.4月(95%CI: 6.23~8.77)。最常见的严重不良反应为中性粒细胞减少、贫血、白细胞减低、腹泻、恶心、呕吐等,需警惕严重的中性粒细胞减少、贫血、白细胞减低及严重腹泻的发生。
结论Sacituzumab govitecan可为晚期HER2阴性乳腺癌患者带来显著的生存获益,使用过程中需警惕严重不良反应的发生。
-
关键词:
- 乳腺癌 /
- Sacituzumab govitecan /
- 临床试验 /
- Meta分析
Abstract:ObjectiveTo explore the efficacy and safety of Sacituzumab govitecan on patients with advanced HER2-negative breast cancer.
MethodsWe classified and analyzed the relevant phase Ⅱ or Ⅲ clinical trials published by May 2021, and used the Stata 12.0 software package for analyzing efficacy and toxicity of Sacituzumab govitecan on the patients with advanced HER2-negative breast cancer.
ResultsThree studies involving 420 patients with advanced HER2-negative breast cancer were included. Among them, one randomized controlled trial (RCT) study and two cohort studies were conducted. Meta-analysis results showed that total objective response rate of breast cancer to Sacituzumab govitecan was 34%(95%CI: 29.4%-38.6%), and the clinical benefit rate was 45.0%(95%CI: 40.2%-49.8%), the median progression-free survival was 5.59 months (95%CI: 4.95-6.31), the median overall survival was 12.59 months (95%CI: 11.58-13.68), and the median duration of response was 7.40 months(95%CI: 6.23-8.77). The most common serious adverse reactions were neutropenia, anemia, leukopenia, diarrhea, nausea, vomiting, etc. The serious neutropenia, anemia, leukopenia and severe diarrhea should be vigilant.
ConclusionSacituzumab govitecan can bring significant survival benefits to patients with advanced HER2-negative breast cancer, and severe adverse reactions should be vigilant during use procedure.
-
Key words:
- Breast cancer /
- Sacituzumab govitecan /
- Clinical trial /
- Meta analysis
-
0 引言
胃癌是世界范围内最常见的恶性肿瘤之一,据2019年发布的全球癌症统计报告结果显示,全球胃癌发病例数约103.4万,死亡例数为75.3万,分别居全球第五位和第三位,居我国第三位和第二位[1-2]。胃癌的早期诊治与预后密切相关,同时其转移、复发及耐药性与疗效及预后密切相关[3]。传统的治疗手段效果并不显著,5年生存率仅为40%,而伴有远处转移的患者5年生存率低于5%[4],进展期胃癌患者的中位生存时间仅11月左右[5]。近年来,以肺癌、乳腺癌和结肠癌[6-8]为代表的实体肿瘤中,分子靶向治疗取得了较好疗效。不同miRNA的靶基因表达量也不同[9]。说明胃癌的发生与miRNA、靶基因密切相关,本研究旨在挖掘伴随胃癌发生相关的miRNA与靶基因,从新的角度识别核心基因和miRNA。
1 材料与方法
1.1 实验材料
人胃癌SGC-7901细胞株(由吉林大学白求恩医学院生命科学院实验室馈赠,本实验室冻存储备)、NCBI、Targetscan、StarBase和miRBase数据库。
1.2 主要试剂
miR-7-5p mimics、miR-7-5p mimics NC、miR-7-5p inhibitor、miR-7-5p inhibitor NC和转染试剂购自苏州吉玛基因股份有限公司;注射用奥沙利铂(OXA)购自江苏恒瑞医药股份有限公司;兔抗人Bax、Bcl-2、caspase-3、caspase-9一抗购自中国武汉爱博泰克生物科技有限公司;辣根过氧化物酶标记的山羊抗兔二抗购自北京中杉金桥生物技术有限公司;DMEM高糖培养基购自美国Hyclone公司;胎牛血清购自浙江天杭生物科技股份有限公司;噻唑蓝染液(MTT)、超敏ECL化学发光试剂盒和二甲基亚砜(DMSO)购自北京鼎国昌盛生物技术有限公司;荧光定量PCR试剂盒购自北京全式金生物技术有限公司;Hoechst33258染色液购自中国碧云天生物技术有限公司;流式细胞周期检测试剂盒购自美国BD公司。
1.3 主要仪器
超净工作台购自美国Thermo Forma公司;Strata Gene Mx3000P荧光实时定量分析系统购自美国Agilent Technologies公司;mini-protean小垂直板电泳系统购自美国Bio-Rad Laboratories Inc公司;Molecular Devices; E-CLIPSE 80i显微镜购自日本Nikon Instruments Inc公司;CO2恒温培养箱购自美国SIM公司。
1.4 细胞体外培养
SGC-7901细胞于含10%胎牛血清的DMEM高糖培养基进行培养,置于37℃、5%CO2培养箱中,两天换液一次,0.25%胰蛋白酶消化,每周传代3次,取对数生长期细胞用于实验。
1.5 生物信息学方法
通过NCBI-pubmed数据库检索出MAPK家族与胃癌细胞凋亡相关的基因和miRNA,生物信息学软件TargetScan、StarBase和miRBase数据库进行互靶验证并取交集,找到互靶关系良好的miRNA与靶基因,通过DAVID分析中GO分析与KEGG通路富集,找到评分较高保守性良好的作为研究对象。
1.6 Real-time PCR检测转染是否成功及时间节点
待细胞单层占孔底的70%~80%时进行转染,8 h更换有血清培养液培养,24 h后收样,提取各组SGC-7901细胞miRNA,配置反转录反应液,反转录合成cDNA,配成20 μl的稳定体系,37℃水浴锅孵育60 min,85℃加热5 s失活RT Enzyme Mix。采用Real-time PCR试剂盒,配置20 μl的Real-time PCR反应体系,检测Ct值,利用2-ΔΔCt公式计算miR-7-5p的相对表达量,引物序列见表 1。分别计算24、36、48 h miR-7-5p的相对表达量。实验重复三次。
表 1 Real-time PCR检测基因的引物序列Table 1 Primer sequences detected by Real-time PCR
1.7 实验及药物处理分组
奥沙利铂(OXA),4℃保存,实验时现用现配,用无血清培养液稀释。实验分为六组:空白对照组(药物浓度为0 μmol/L)、过表达组(miR-7-5p mimics)、过表达阴性对照组(miR-7-5p mimics NC)、沉默组(miR-7-5p inhibitor)、沉默阴性对照组(miR-7-5p inhibitor NC)和OXA组(药物浓度33 μmol/L)。
1.8 实验方法
1.8.1 Real-time PCR验证miR-7-5p与RAF1靶向关系
一块6孔板分别加入1 ml无血清培养液和1 ml RAF1抑制剂(6 nmol/L浓度处理),另一块6孔板中三孔分别加miR-7-5p mimics、miR-7-5p mimics NC为5 μl和无血清培养液,提取细胞总RNA,反转录生成cDNA,Real-time PCR实验分析,测出相应的Ct值。利用2-ΔΔCt公式计算mRNA的相对表达量,GAPDH作为内参。实验重复三次。
1.8.2 MTT法检测细胞增殖能力
每孔200 μl接种于96孔细胞培养板中,每组设7个复孔,培养44 h,吸去培养液,每孔加入MTT溶液(5 mg/ml)200 μl,继续培养4 h,小心吸去MTT溶液,按照每孔150 μl加入DMSO,摇床上摇晃15 min,使结晶充分溶解,于酶标仪上检测490 nm波长检测相应的OD值。实验重复三次。
1.8.3 Hoechst33258核染色法检测细胞凋亡形态
收样后使用Hoechst33258染色液染色,抗荧光淬灭封片液封片后,于荧光显微镜下观察细胞凋亡形态变化情况,Images Advanced3.2成像系统采集图像,实验重复三次。
1.8.4 流式细胞术检测细胞周期
收样,1000 r/min离心5 min,弃培养液,1 ml PBS洗涤后,加入预冷的70%乙醇溶液,4℃固定至少18 h,离心弃固定液,PBS清洗一次,弃PBS,加入0.5 ml PI染液,37℃避光孵育30 min后重悬细胞,1 h内流式细胞仪检测细胞周期,实验重复3次。
1.8.5 划痕愈合实验检测细胞迁移能力
细胞单层融合度接近100%孔底时,用200 μl的黄枪头垂直在细胞上划线,PBS洗去漂浮的细胞,转染加药,放置于37℃的CO2恒温培养箱中培养48 h,显微镜下观察细胞划痕愈合情况,拍照保存,实验重复三次。
1.8.6 Western blot检测相关分子蛋白表达水平
收样后,经消化、洗涤、裂解、煮沸、离心,收集蛋白,上样,进行SDS-PAGE凝胶电泳,湿转法转膜至PVDF膜上,5%的脱脂奶粉封闭1 h,一抗(1:2 000)4℃孵育过夜,TBST洗膜后加入对应的二抗(1:5 000),超敏ECL化学发光试剂盒检测杂交信号,Image Quant LAS 4000 Mini凝胶成像仪自动曝光,分析灰度值,实验重复三次。
1.9 统计学方法
采用SPSS22.0处理数据,数据符合正态分布并经方差齐性检验,数据以(x±s)表示,不同组间miR-7-5p的表达差异比较使用t检验,两两之间比较采用LSD检验,P < 0.05为差异有统计学意义。
2 结果
2.1 生物信息学实验结果
通过NCBI-pubmed进行文献检索,共筛选出27个miRNA和66个基因存在于MAPK家族当中并与人类胃癌紧密相关,且具有显著的作用。
2.1.1 胃癌相关miRNA靶基因的预测
利用生物信息学软件TargetScan、StarBase和miRBase数据库进行靶向关系验证并取交集,有四对互靶关系评分较高且保守性良好,见表 2。
表 2 基因与miRNA的相互靶标关系Table 2 Targeting relation between gene and miRNA
2.1.2 胃癌相关miRNA靶基因的功能
发现有且仅有miR-7-5p—RAF1这一对存在于MAPK家族之中,RAF1可能通过其674-681位碱基与miR-7-5p的3’UTR高度互补结合,见图 1。
2.2 Real-time PCR检验转染是否成功、转染时间节点及miR-7-5p与RAF1靶向关系
miR-7-5p在胃癌SGC-7901细胞中的表达显著降低(P=0.001),见图 2A。miR-7-5p mimics与Control和miR-7-5p mimics NC组相比显著升高(P=0.029),证明转染成功,见图 2B。转染24、36和48 h,miR-7-5p在胃癌SGC-7901细胞中的表达逐渐升高,故将48 h作为转染的时间结点,见图 2C。RAF1在胃癌SGC-7901细胞中的表达上调(见Control组),对miR-7-5p过表达后RAF1呈下降趋势(P=0.002),见图 3A,且使用RAF1抑制剂后,miR-7-5p的相对表达量明显升高(P=0.048),见图 3B。说明miR-7-5p与RAF1存在互靶关系。
2.3 OXA、miR-7-5p过表达及沉默对各组细胞的生长抑制情况
miR-7-5p过表达(miR-7-5p mimics)、沉默(miR-7-5p inhibitor)及给药处理48 h后,与对照组相比,miR-7-5p mimics和OXA组抑制胃癌SGC-7901细胞的生长,且miR-7-5p mimics组抑制作用更显著(P=0.004,P=0.002),miR-7-5p inhibitor促进胃癌SGC-7901细胞的生长(P=0.045),见表 3。
表 3 OXA和miR-7-5p mimics组抑制胃癌SGC-7901细胞的生长(x±s, n=7)Table 3 OXA and miR-7-5p mimics group inhibited the growth of gastric cancer SGC-7901 cells (x±s, n=7)
2.4 细胞凋亡的形态学观察
转染及给药处理48 h后,经过Hoechst33258染色,荧光显微镜下显示,正常细胞的细胞核呈弥散均匀蓝色荧光,而凋亡细胞的细胞核浓染致密,或呈颗粒碎块状,颜色发白发亮。miR-7-5p mimics和OXA组均可见到此种凋亡细胞的表现,见图 4。
2.5 miR-7-5p及OXA对胃癌细胞周期的影响
转染及给药处理48 h后,与对照组相比,miR-7-5p mimics组与OXA组周期进程减慢,G0/G1期百分率增加,G2/M期百分率降低,说明miR-7-5p mimics与OXA均具有减慢细胞周期从而抑制胃癌细胞SGC-7901增殖的作用,而miR-7-5p inhibitor作用相反(P=0.002, P=0.000),见图 5。
2.6 细胞划痕愈合情况
转染及给药处理48 h后,与Control组相比,miR-7-5p inhibitor组侵袭转移能力增强(P=0.001),而miR-7-5p mimics与OXA组细胞侵袭转移能力降低(P=0.022),见图 6。
2.7 相关分子蛋白含量检测结果
Western blot实验灰度值分析表明:与Control组相比,miR-7-5p mimics及OXA可显著上调caspase-3、caspase-9、Bax及下调Bcl-2、p-RAF1的蛋白表达水平(均P < 0.05), 且呈剂量相关性,见图 7。
![]() 图 7 Western blot检测SGC-7901细胞中Bcl-2、Bax、Cleaved-caspase-3、Cleaved-caspase-9、RAF1和p-RAF1的表达Figure 7 Bcl-2, Bax, Cleaved-caspase-3, Cleaved-caspase-9, RAF1 and p-RAF1 expression in SGC-7901 cells detected by Western blot1: miR-7-5p mimics; 2: miR-7-5p mimics NC; 3: miR-7-5p inhibitor; 4: miR-7-5p inhibitor NC; 5: OXA; 6: Control.
图 7 Western blot检测SGC-7901细胞中Bcl-2、Bax、Cleaved-caspase-3、Cleaved-caspase-9、RAF1和p-RAF1的表达Figure 7 Bcl-2, Bax, Cleaved-caspase-3, Cleaved-caspase-9, RAF1 and p-RAF1 expression in SGC-7901 cells detected by Western blot1: miR-7-5p mimics; 2: miR-7-5p mimics NC; 3: miR-7-5p inhibitor; 4: miR-7-5p inhibitor NC; 5: OXA; 6: Control.3 讨论
microRNA(miRNA)是一种在真核生物中普遍存在的具有调控功能的内源性小分子RNA。自1993年首次在秀丽隐杆线虫体内发现miRNA编码基因-lin-4基因之后[10],相继在果蝇、植物以及人的多种组织中发现了它的存在[11]。正常细胞中,miRNA通过与靶基因mRNA特异性结合,促使靶基因mRNA的降解或者翻译抑制,从而调节细胞的增殖、分化以及凋亡等多种生理学过程。而在肿瘤细胞中,某些miRNA可以影响正常的信号转导,通过调控下游基因的表达来调节细胞增殖、分化以及凋亡等生理学过程,进而在肿瘤发生发展中起到类似促癌基因或抑癌基因的作用[12]。miR-7位于第15号染色体上,是一种高度保守的小分子RNA,miR-7与癌症的发生发展有着非常紧密的关系,在多种肿瘤细胞中呈异常表达,如神经肿瘤、宫颈癌、膀胱癌和肝癌等[13-16]。作为miR-7的重要分支,miR-7-5p发挥着非常重要的作用。
奥沙利铂作为临床上常用的抗肿瘤药物,在疗效方面发挥着重要的作用,虽主要作用于患者肿瘤病灶,但长期使用会造成血液毒性、肾毒性、耳毒性及全身损害等严重不良反应[17-18],寻找可以替代的低毒药物或者分子层面的治疗成为了一个新思路。本实验结果显示,过表达组与奥沙利铂组均会促进胃癌SGC-7901细胞的凋亡,减慢细胞周期进程,将大量细胞维持在G0/G1期。Cleaved-caspase3和Cleaved-caspase9蛋白表达量显著升高,当caspase家族被激活后,在caspase前体的N-端前肽和大亚基之间的特定位点被水解去除N-端前肽,然后在大小亚基之间切割释放大小亚基,进而形成两两组成的有活性的异四聚体。此时caspase3对执行者caspase9进行切割并使之激活,caspase9通过对caspase靶蛋白的水解,导致细胞发生凋亡。说明miR-7-5p mimics与OXA均可促进胃癌细胞的凋亡,且miR-7-5p mimics与OXA组间差异无统计学意义,在抑制胃癌细胞的增殖方面无明显差异。本实验通过前期生物信息学理论基础与Real-time PCR实验验证,miR-7-5p与RAF1存在互靶关系,且有证据表明,荧光素酶活性测定实验鉴定RAF1是miR-7-5p的直接靶点[19]。Western blot结果也显示miR-7-5p与RAF1含量呈现显著负相关。
综上所述,miR-7-5p可以通过过表达或沉默靶基因RAF1,与化疗药物奥沙利铂同样起到抑制胃癌细胞的作用,提示奥沙利铂可能通过上调miRNA-7-5p促进胃癌细胞SGC-7901的凋亡、减慢侵袭转移速度。低毒高效的miR-7-5p在未来分子层面的治疗有可能取代奥沙利铂。
Competing interests: The authors declare that they have no competing interests.作者贡献:张倩:文献检索、筛选,数据分析和文章撰写李娜:文献检索、筛选和文章撰写杨华:研究设计、文章修改 -
表 1 纳入研究的基本特征
Table 1 Baseline characteristics of eligible studies

表 2 接受Sacituzumab govitecan治疗患者的不良反应(n(%))
Table 2 Adverse events in patients receiving Sacituzumab govitecan treatment (n(%))

-
[1] Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. doi: 10.3322/caac.21660
[2] Harb WA. Management of patients with hormone receptor-positive breast cancer with visceral disease: challenges and treatment options[J]. Cancer Manag Res, 2015, 7: 37-46.
[3] BrufskyA, Valero V, Tiangco B, et al. Second-line bevacizumab-containing therapy in patients with triple-negative breast cancer: subgroup analysis of the RIBBON-2trial[J]. Breast Cancer Res Treat, 2012, 133(3): 1067-1075. doi: 10.1007/s10549-012-2008-6
[4] Goldenberg DM, Sharkey RM. Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy[J]. Expert Opin Biol Ther, 2020, 20(8): 871-885. doi: 10.1080/14712598.2020.1757067
[5] Sharkey RM, Govindan SV, Cardillo TM, et al. Selective and Concentrated Accretion of SN-38 with a CEACAM5-Targeting Antibody-Drug Conjugate (ADC), Labetuzumab Govitecan (IMMU-130)[J]. Mol Cancer Ther, 2018, 17(1): 196-203. doi: 10.1158/1535-7163.MCT-17-0442
[6] Starodub AN, Ocean AJ, Shah MA, et al. First-in-Human Trial of a Novel Anti-Trop-2 Antibody-SN-38 Conjugate, Sacituzumab Govitecan, for the Treatment of Diverse Metastatic Solid Tumors[J]. Clin Cancer Res, 2015, 21(17): 3870-3878. doi: 10.1158/1078-0432.CCR-14-3321
[7] Rugo HS, Bardia A, Tolaney SM, et al. TROPiCS-02: A Phase Ⅲ study investigating Sacituzumab govitecan in the treatment of HR+/HER2- metastatic breast cancer[J]. Future Oncol, 2020, 16(12): 705-715. doi: 10.2217/fon-2020-0163
[8] Dent O. Methodological index for non-randomized studies[J]. ANZJ Surg, 2003, 73(9): 675-676. doi: 10.1046/j.1445-2197.2003.02762.x
[9] Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer[J]. N Engl J Med, 2019, 380(8): 741-751. doi: 10.1056/NEJMoa1814213
[10] Kalinsky K, Diamond JR, Vahdat LT, et al. Sacituzumab govitecan in previously treated hormone receptor-positive/HER2-negative metastatic breast cancer: final results from a phase Ⅰ/Ⅱ, single-arm, basket trial[J]. Ann Oncol, 2020, 31(12): 1709-1718. doi: 10.1016/j.annonc.2020.09.004
[11] Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer[J]. N Engl J Med, 2021, 384(16): 1529-1541. doi: 10.1056/NEJMoa2028485
[12] Park IH, Lee KS, Ro J. Effects of second and subsequent lines of chemotherapy for metastatic breast cancer[J]. Clin Breast Cancer, 2015, 15(1): e55-e62. doi: 10.1016/j.clbc.2014.09.001
[13] Zeichner SB, Terawaki H, Gogineni K. A review of systemic treatment in metastatic triple-negative breast cancer[J]. Breast Cancer (Auckl), 2016, 10: 25-36.
[14] Shvartsur A, Bonavida B. Trop2 and its overexpression in cancers: regulation and clinical/therapeutic implications[J]. Genes Cancer, 2015, 6(3-4): 84-105.
[15] Vidula N, Yau C, Rugo HS. Trop2 gene expression (Trop2e) in primary breast cancer (BC): Correlations with clinical and tumor characteristics[J]. J Clin Oncol, 2017, 35(15_suppl): 1075. doi: 10.1200/JCO.2017.35.15_suppl.1075
[16] 国家肿瘤质控中心乳腺癌专家委员会, 中国抗癌协会乳腺癌专业委员会, 中国抗癌协会肿瘤药物临床研究专业委员会. 中国晚期乳腺癌规范诊疗指南(2020版)[J]. 中华肿瘤杂志, 2020, 42(10): 781-797. doi: 10.3760/cma.j.cn112152-20200817-00747 National Cancer Quality Control Center Breast Cancer Expert Committee, China Cancer Association Breast Cancer Specialized Committee, China Cancer Association Clinical Research of Cancer Medicine Specialized Committee. Guidelines for clinical diagnosis and treatment of advanced breast cancer in China (2020 Edition)[J]. Zhonghua Zhong Liu Za Zhi, 2020, 42(10): 781-797. doi: 10.3760/cma.j.cn112152-20200817-00747
[17] Kalinsky K, Oliveira M, Traina TA, et al. Outcomes in patients (pts) aged≥65 years in the phase 3 ASCENT study of sacituzumab govitecan (SG) in metastatic triple-negative breast cancer (mTNBC)[J]. J Clin Oncol, 2021, 39(15 suppl): 1011.
[18] Bardia A, Messersmith WA, Kio EA, et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase Ⅰ/Ⅱ IMMU-132-01 basket trial[J]. Ann Oncol, 2021, 32(6): 746-756. doi: 10.1016/j.annonc.2021.03.005
[19] Shen M, Liu S, Stoyanova T. The role of Trop2 in prostate cancer: an oncogene, biomarker, and therapeutic target[J]. Am J Clin Exp Urol, 2021, 9(1): 73-87.
-
期刊类型引用(1)
1. 李云霞. 非小细胞肺癌患者外周血CTC与临床特征的关系及其临床意义. 航空航天医学杂志. 2021(05): 543-545 .  百度学术
百度学术
其他类型引用(2)



 下载:
下载: