Efficacy and Safety of Apatinib Monotherapy as Subsequent-line Therapy on Patients with Advanced Esophageal Squamous Cell Carcinoma
-
摘要:目的
探讨阿帕替尼后线治疗晚期ESCC患者的疗效和安全性。
方法纳入56例后线接受阿帕替尼单药治疗的晚期ESCC患者。阿帕替尼治疗的起始剂量为每天500 mg或250 mg。分析患者的临床病理资料、不良反应及预后。研究主要终点为无进展生存期(PFS),次要终点为客观缓解率(ORR)、疾病控制率(DCR)、总生存期(OS)和接受阿帕替尼治疗的安全性。
结果56例ESCC患者均符合入选标准且均可评价疗效与不良反应。阿帕替尼单药后线治疗晚期ESCC患者的ORR为8.9%(95%CI: 3.0%~19.6%),DCR为64.3%(95%CI: 50.4%~76.6%)。中位PFS为3.7月(95%CI: 3.19~4.21),中位OS为6.3月(95%CI: 3.53~9.08)。晚期ESCC患者后线接受阿帕替尼治疗过程中相对常见的不良反应有高血压(50.0%)、乏力(41.1%)、食欲下降(35.7%)、手足综合征(30.4%)和腹泻(26.8%)等。
结论阿帕替尼单药在晚期ESCC患者后线治疗中具有潜在的疗效及可耐受的安全性。但研究结果尚需要前瞻性临床研究进一步验证。
Abstract:ObjectiveTo investigate the efficacy and safety of apatinib monotherapy as subsequent-line therapy on patients with advanced ESCC.
MethodsWe included 56 patients with advanced ESCC who were administered with apatinib monotherapy. The initial dosage of apatinib was 500mg or 250mg daily. Clinicopathological characteristics, adverse reaction and prognosis of the patients were analyzed. The primary endpoint of this study was PFS, the secondary endpoints were ORR, DCR, OS and safety of apatinib administration.
ResultsAll the 56 patients with ESCC corresponded with the eligibility criteria and were available for the evaluation of efficacy and adverse reaction. The ORR of the 56 patients who received apatinib monotherapy was 8.9% (95%CI: 3.0%-19.6%) and DCR was 64.3% (95%CI: 50.4%-76.6%). The median PFS was 3.7 months (95%CI: 3.19-4.21) and the median OS was 6.3 months (95%CI: 3.53-9.08). The common adverse reactions were hypertension (50.0%), fatigue (41.1%), loss of appetite (35.7%), hand-foot syndrome (30.4%) and diarrhea (26.8%).
ConclusionApatinib monotherapy demonstrates potential efficacy and tolerable safety as the further-line treatment for the patients with advanced ESCC. And the conclusion should be validated in prospective clinical studies subsequently.
-
Key words:
- Esophageal squamous cell carcinoma /
- Apatinib /
- Efficacy /
- Prognosis /
- Safety
-
0 引言
目前我国食管癌每年新发病例32.0万,新增死亡病例15.7万[1]。西方国家主要以食管腺癌为主,我国约95%的食管癌患者是食管鳞癌(esophageal squamous cell carcinoma, ESCC)[2]。目前手术治疗仍然是ESCC最主要的治疗方式。然而对于无手术切除机会的ESCC患者,同步放化疗和化疗方案也为患者带来了一定的生存获益[3]。但在转移性的ESCC患者中,放疗的价值已经非常有限,目前临床上主要是以顺铂联合5-Fu或紫杉醇为基础的化疗[4]。一线化疗方案中的顺铂联合5-Fu的方案在转移性ESCC中可以取得约33%的客观缓解率(objective response rate, ORR)和5.5月的中位无进展生存期(progression free survival, PFS)以及10月的中位总生存期(overall survival, OS)[5]。而顺铂联合紫杉醇的方案在转移性ESCC患者中的ORR约为42.5%,中位PFS和OS分别为7月和13月[6]。虽然靶向治疗药物如尼妥珠单抗等在一线治疗中联合化疗取得了一定的疗效,但是较高级别的循证医学证据尚有限[7]。
晚期ESCC一线治疗进展后标准治疗为单药化疗方案。伊立替康、多西他赛、紫杉醇均为二线可用的化疗方案。这些单药方案的有效率约为7.4%,中位PFS和OS分别为3月和7.1月[8]。此外,免疫检查点抑制剂在二线治疗中也取得了阳性结果,其中pembrolizumab、nivolumab和卡瑞利珠单抗均在晚期ESCC的二线治疗中进一步给患者带来生存获益[9]。然而,在晚期ESCC后线治疗中目前尚无标准的治疗方案,患者既往接受前线治疗进展后急需有效的治疗药物。阿帕替尼是主要作用于VEGFR2靶点的抗血管生成小分子TKI,相关的Ⅱ期临床研究结果表明阿帕替尼在晚期ESCC后线中具有初步的疗效及安全性[10]。然而,目前在临床实践中阿帕替尼在晚期ESCC患者后线治疗中的疗效及安全性数据尚且缺乏。因此,本研究旨在探讨真实世界中阿帕替尼单药在晚期ESCC患者后线治疗中的疗效及安全性。
1 资料与方法
1.1 研究设计及入排标准
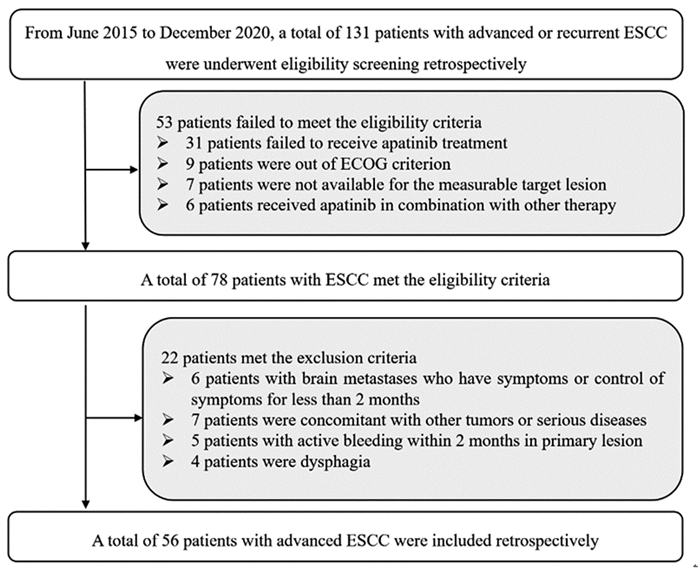
回顾性分析2015年6月—2020年12月在郑州人民医院放疗科和肿瘤科接受治疗的晚期ESCC患者的临床资料。纳入标准:(1)经组织学或细胞学确诊的ESCC,且为转移性或复发性的ESCC;(2)东部肿瘤协作组(Eastern Cooperative Oncology Group, ECOG)体质状况评分为0~2分;(3)按照RECIST 1.1标准具有可测量的病灶;(4)既往接受过标准的系统性的治疗方案出现疾病进展或不耐受,系统治疗包括同步放化疗或化疗方案;(5)患者接受阿帕替尼单药的治疗。排除标准:(1)伴有症状或疾病控制时间少于两月的脑转移患者;(2)原发病灶两月内有活动性出血的ESCC患者;(3)吞咽功能障碍的患者;(4)合并其他可能影响患者生存的恶性肿瘤或者严重疾病的患者。本研究流程见图 1,最终56例ESCC患者符合研究筛选标准。本研究的主要终点为PFS,次要终点为ORR、DCR、OS和安全性。
56例ESCC患者中,35例患者(62.5%)一线治疗接受铂类药物联合紫杉醇或多西他赛方案(其中双药化疗联合尼妥珠单抗或西妥昔单抗患者4例)、12例患者(21.4%)一线治疗接受铂类药物联合5-Fu方案、9例患者(16.1%)一线治疗接受奥沙利铂联合卡培他滨或替吉奥方案。二线治疗方案中,接受单药化疗(伊立替康或紫杉醇或多西他赛或替吉奥或吉西他滨)治疗的患者43例(76.8%)、接受PD-1单抗治疗的患者8例(14.3%)、接受EGFR-TKI治疗的患者5例(8.9%)。44例患者三线及以上治疗接受化疗单药患者32例(72.7%)、接受中药抗肿瘤治疗患者8例(18.2%)、接受PD-1治疗患者4例(9.1%)。入组时患者治疗进展的部位分别为淋巴结25例(44.6%)、肺部病灶14例(25.0%)、原发灶9例(16.1%)、肝部病灶8例(14.3%)。
1.2 疗效及安全性评价
研究纳入的56例ESCC患者均接受阿帕替尼单药治疗。具体用法用量:阿帕替尼起始剂量为500 mg每天或250 mg每天(根据患者的体质状态由研究者综合决定阿帕替尼剂量),餐后半小时服用,直至疾病进展或出现不可耐受的不良反应。根据治疗过程中出现的血液学或者非血液学毒性调整相应的剂量,一旦发生可能威胁生命的不良反应时治疗中止。本研究通过郑州人民医院伦理委员会批准。
采用RECIST(1.1版本)标准进行患者的疗效评估[11],分为:完全缓解(CR)、部分缓解(PR)、疾病稳定(SD)和疾病进展(PD),ORR为CR和PR患者的比例。DCR为CR、PR及SD患者的比例。每两个月或者根据患者实际的临床症状通过影像学的计算机断层扫描(CT)或核磁共振成像(MRI)方法对靶病灶的变化进行评价,记录患者每次影像学评估检查结果。本研究ORR和DCR的计算均采用患者接受治疗过程中最佳的疗效评估结果进行分析。阿帕替尼治疗过程中出现的药物相关不良反应通过美国国立癌症研究所的常见不良反应标准(CTCAE v5.0版本)进行评价。
患者住院接受阿帕替尼治疗期间的疗效及安全性可以通过医院电子病历系统获取。患者疾病进展后的随访主要通过电话方式进行,每月和患者进行一次电话访视获取患者出现疾病进展及后续的治疗情况。
1.3 统计学方法
所有数据均采用SPSS25.0统计软件进行分析。PFS和OS数据采用Stata14.0软件绘制Kaplan-Meier曲线。PFS定义为患者开始接受阿帕替尼治疗的日期至出现肿瘤进展或者死亡的日期,OS定义为患者开始接受阿帕替尼治疗的日期至因任何原因导致死亡的日期。最后一次随访时尚未出现疾病进展或死亡的,则按照删失数据进行处理。P < 0.05为差异有统计学意义。
2 结果
2.1 患者基线临床资料
本研究纳入的56例接受阿帕替尼单药治疗的晚期ESCC患者基线临床资料见表 1。
表 1 56例既往经治的晚期食管鳞癌患者的基线临床资料Table 1 Baseline characteristics of 56 patients with previously- treated advanced ESCC
2.2 阿帕替尼单药治疗晚期ESCC患者的疗效
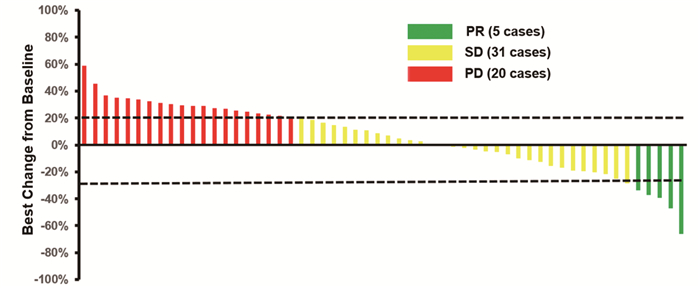
56例患者均可评价疗效。其中CR 0例,PR 5例,SD 31例,PD 20例。ORR为8.9%(95% CI: 3.0%~19.6%),DCR为64.3%(95%CI: 50.4%~76.6%)。针对靶病灶大小的最佳百分比变化的瀑布图见图 2。
2.3 预后
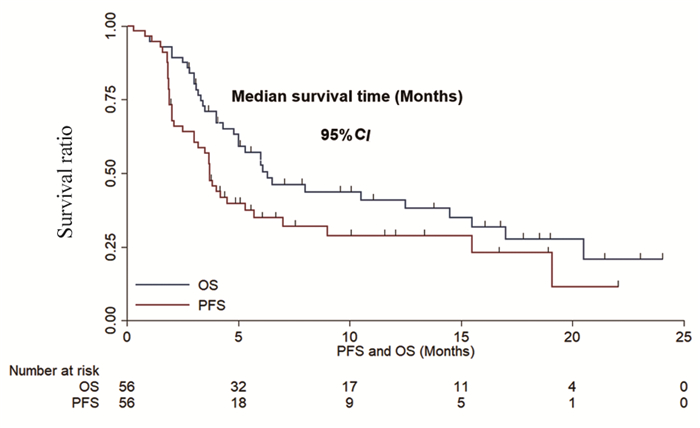
本研究最后一次随访时间为2021年3月30日,中位随访时间为6.1月(0.3~24月)。截至随访时间,39例(69.6%)患者出现PFS或死亡事件,中位PFS为3.7月(95%CI: 3.19~4.21);35例(62.5%)患者出现OS事件,中位OS为6.3月(95%CI: 3.53~9.08),见图 3。
对56例晚期ESCC患者进行PFS的单因素分析,结果显示:只有ECOG评分和年龄与PFS有显著相关性。对单因素分析中和PFS有差异的变量纳入多因素分析,结果显示:经多变量校正,年龄(HR=0.73, P=0.041)和ECOG评分(HR=0.57, P=0.022)对PFS仍然具有独立的影响,见表 2。
表 2 56例晚期食管鳞癌患者中根据基线临床资料的针对PFS的单变量和多变量分析Table 2 PFS of 56 patients with advanced ESCC according to baseline characteristics in univariate and multivariate analyses
2.4 不良反应发生情况
56例晚期ESCC患者接受阿帕替尼治疗过程中可能和药物相关的不良反应发生情况,见表 3。阿帕替尼单药治疗期间不良反应整体安全可控,本研究未观察到5级不良反应的发生,常见的不良反应主要有高血压、疲劳、食欲下降、手足综合征(HFS)、腹泻、恶心呕吐、蛋白尿、血液学毒性和出血。其中,常见的3~4级不良反应有高血压(16.1%)、HFS(14.3%)、乏力(5.4%)、食欲下降(3.6%)、血液学毒性(3.6%)、恶心呕吐(1.8%)和出血(1.8%)。
表 3 接受阿帕替尼单药治疗的56例晚期食管鳞癌患者中的不良反应发生情况(n(%))Table 3 Adverse reaction of 56 advanced ESCC patients receiving apatinib monotherapy (n(%))
3 讨论
食管癌作为一种异质性较强的消化系统恶性肿瘤,在中国人群中的发病率逐年上升。ESCC是我国最常见的类型,所以很多国外的在食管腺癌中的研究数据并不适用于中国的ESCC患者[12]。经典的铂类联合5-Fu或紫杉醇的方案在临床上已经使用超过20年,靶向药物在晚期ESCC中的探索均未取得阳性结果[13]。然而,从2019年开始免疫检查点抑制剂在晚期ESCC中逐步取得突破性进展。最早的Keynote181研究和Attraction 3研究分别奠定了pembrolizumab和nivolumab在晚期ESCC患者二线治疗中的地位,不过这两个研究纳入的并不完全是ESCC患者,价值相对有限[14]。ESCORT研究则在中国ESCC患者中开展,证实了卡瑞利珠单抗在晚期ESCC患者二线治疗中的地位[9]。然而,在晚期ESCC后线治疗中目前尚无标准方案。安罗替尼作为小分子TKI逐步填补了这方面的治疗空白[15]。
本研究中56例接受阿帕替尼治疗的晚期ESCC患者的ORR为8.9%,DCR为64.3%,中位PFS为3.7月,和Chu教授团队[16]开展的Ⅱ期临床研究结果基本一致。该研究纳入40例标准治疗方案失败后接受阿帕替尼治疗的晚期ESCC患者,结果表明阿帕替尼组患者的ORR为7.5%,DCR为65.0%,中位PFS为3.8月。此外,Yanwei教授团队[10]的Ⅱ期临床研究也入组了32例接受阿帕替尼单药的治疗复发转移性ESCC患者,结果表明接受阿帕替尼治疗患者的ORR为7.7%,DCR为61.5%,这和本研究的结果基本一致。不过,他们的研究发现患者的中位PFS为4.63月,高于本研究结果。原因可能和他们研究入组的样本量相对较小、PFS数据可能存在一定的偏倚有关,也可能是因为他们设计的是前瞻性临床研究,患者管理及患者依从性都要比回顾性研究更严谨[17]。此外,另一个抗血管生成的小分子TKI安罗替尼也被CSCO食管癌指南推荐为食管鳞癌后线治疗药物。安罗替尼的Ⅱ期临床研究纳入165例一线治疗失败的ESCC患者分别接受安罗替尼和安慰剂的治疗。结果表明安罗替尼治疗组患者的ORR为7.34%,DCR为64.22%,中位PFS为3.02月[18],和本研究中阿帕替尼后线治疗ESCC患者的疗效及PFS基本一致。这也表明了抗血管生成TKI在ESCC患者中具有潜在的临床治疗意义。此外,由于本研究随访时间相对较长,因此也进行了OS的评价。结果表明56例接受阿帕替尼单药治疗的晚期ESCC患者的中位OS为6.3月,略优于以上Ⅱ期临床研究中的5.8月。我们推测可能因近两年来随着免疫治疗药物陆续上市,更多的ESCC患者在前线或后线治疗中都可以有机会接受PD-1/PD-L1抑制剂的治疗。本研究中有12例患者既往接受过免疫抑制剂的治疗。此外,也有其他的抗血管生成小分子TKI,比如安罗替尼,被证实可以给晚期ESCC患者带来生存获益[18]。实际上,本研究中的部分患者在阿帕替尼治疗进展后又接受了免疫药物或其他的抗血管生成小分子药物的治疗,这在一定程度上都给患者带来了生存获益。
阿帕替尼单药治疗的不良反应主要有高血压、疲劳、食欲下降、手足综合征(HFS)、腹泻、恶心呕吐、蛋白尿、血液学毒性和出血,没有出现非预期的不良反应。这和之前的Ⅱ期临床研究中的常见不良反应类型基本一致[10, 16]。不过整体的发生率要略低于Ⅱ期临床研究中的不良反应发生率,原因可能是本研究中62.5%的患者接受阿帕替尼的剂量为250 mg,而临床研究中阿帕替尼的剂量均为500 mg。另外本研究记录到的不良反应大部分是不需要进行实验室检查的不良反应。需要生理生化检查的包括AST/ALT升高以及血液学毒性发生率也略低于临床研究中的发生率。原因主要是因为研究设计为回顾性分析,部分患者未及时地进行相关的生理生化检查,因此数据存在缺失和记录不完全,在一定程度上造成了这方面的结果较少且发生率较低。Cheng团队[19]开展的安罗替尼在晚期NSCLC肺癌中的回顾性研究的结果也发现了真实世界中记录到的不良反应发生率低于Ⅲ期研究的情况,主要是因为回顾性分析对于不良反应的记录相对简单。Li教授团队[20]的回顾性研究探讨了阿帕替尼单药在晚期ESCC患者中的疗效及安全性,结果也发现了不良反应发生率略低于临床研究中的结果,但阿帕替尼的起始剂量是500 mg,这和本研究中阿帕替尼的起始剂量存在一定差异。关于起始剂量的差异是否会影响到患者后续的疗效差异尚需要大样本临床进一步证实。本研究的亚组分析结果未发现500 mg和250 mg起始剂量的患者在PFS方面的差异。年龄≥63岁的患者和ECOG评分为2分的患者接受阿帕替尼治疗后疗效较差,这和既往阿帕替尼在广泛期非小细胞肺癌中的研究结果基本一致[21]。≥63岁的患者和ECOG评分为2分是否能从阿帕替尼治疗中获益的结论尚需要在大样本前瞻性临床研究中进一步验证。
本研究也存在一定的局限性,研究入组的样本量相对较少,结论尚需要在大样本的患者中进行验证。此外,回顾性分析中PFS及OS数据的成熟度相对不高,不良反应的记录也相对不全面,这造成了一些研究的偏倚。不过,本研究总体上探讨了阿帕替尼单药在晚期ESCC患者中的疗效及安全性,研究结果对于晚期ESCC患者标准治疗方案失败后的药物选择具有一定的临床指导意义。
Competing interests: The authors declare that they have no competing interests.作者贡献:侯继院:研究设计、患者管理及论文撰写祁佩红、王海霞:患者管理、随访及数据整理龚哲:统计分析及图表制作单国用:技术支持、数据及论文审核 -
表 1 56例既往经治的晚期食管鳞癌患者的基线临床资料
Table 1 Baseline characteristics of 56 patients with previously- treated advanced ESCC

表 2 56例晚期食管鳞癌患者中根据基线临床资料的针对PFS的单变量和多变量分析
Table 2 PFS of 56 patients with advanced ESCC according to baseline characteristics in univariate and multivariate analyses

表 3 接受阿帕替尼单药治疗的56例晚期食管鳞癌患者中的不良反应发生情况(n(%))
Table 3 Adverse reaction of 56 advanced ESCC patients receiving apatinib monotherapy (n(%))

-
[1] Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2): 115-132. doi: 10.3322/caac.21338
[2] 程娜, 李卫华, 薛丽燕. 食管鳞状细胞癌壁内转移的研究进展[J]. 肿瘤防治研究, 2021, 48(2): 191-195. doi: 10.3971/j.issn.1000-8578.2021.20.0482 Cheng N, Li WH, Xue LY. Progress of intramural metastasis of esophageal squamous cell carcinoma[J]. Zhong Liu Fang Zhi Yan Jiu, 2021, 48(2): 191-195. doi: 10.3971/j.issn.1000-8578.2021.20.0482
[3] Hirano H, Kato K. Systemic treatment of advanced esophageal squamous cell carcinoma: chemotherapy, molecular-targeting therapy and immunotherapy[J]. Jpn J Clin Oncol, 2019, 49(5): 412-420. doi: 10.1093/jjco/hyz034
[4] Kubo K, Wadasaki K, Shinozaki K. Treatment outcomes according to the macroscopic tumor type in locally advanced esophageal squamous cell carcinoma treated by chemoradiotherapy[J]. Jpn J Radiol, 2019, 37(4): 341-349. doi: 10.1007/s11604-019-00814-6
[5] Hayashi K, Ando N, Watanabe H, et al. Phase Ⅱ evaluation of protracted infusion of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG) Trial (JCOG9407)[J]. Jpn J Clin Oncol, 2001, 31(9): 419-423. doi: 10.1093/jjco/hye090
[6] Liu Y, Ren Z, Yuan L, et al. Paclitaxel plus cisplatin vs. 5-fluorouracil plus cisplatin as first-line treatment for patients with advanced squamous cell esophageal cancer[J]. Am J Cancer Res, 2016, 6(10): 2345-2350.
[7] Zhang X, Jia J, Lu M, et al. Nimotuzumab Plus Paclitaxel and Cisplatin as a 1st-Line Treatment for Esophageal Cancer: Long Term Follow-up of a Phase Ⅱ Study[J]. J Cancer, 2019, 10(6): 1409-1416. doi: 10.7150/jca.28659
[8] Kojima T, Shah MA, Muro K, et al. Randomized Phase ⅢKEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer[J]. J Clin Oncol, 2020, 38(35): 4138-4148. doi: 10.1200/JCO.20.01888
[9] Peng C, Cohen DJ. Advances in the pharmacotherapeutic management of esophageal squamous cell carcinoma[J]. Expert Opin Pharmacother, 2021, 22(1): 93-107. doi: 10.1080/14656566.2020.1813278
[10] Yanwei L, Feng H, Ren P, et al. Safety and Efficacy of Apatinib Monotherapy for Unresectable, Metastatic Esophageal Cancer: A Single-Arm, Open-Label, Phase Ⅱ Study[J]. Oncologist, 2020, 25(10): e1464-e1472. doi: 10.1634/theoncologist.2020-0310
[11] Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)[J]. Eur J Cancer, 2009, 45(2): 228-247. doi: 10.1016/j.ejca.2008.10.026
[12] Zhang X, Yang X, Ni J, et al. Recommendation for the definition of postoperative radiotherapy target volume based on a pooled analysis of patterns of failure after radical surgery among patients with thoracic esophageal squamous cell carcinoma[J]. Radiat Oncol, 2018, 13(1): 255. doi: 10.1186/s13014-018-1199-3
[13] Yang YM, Hong P, Xu WW, et al. Advances in targeted therapy for esophageal cancer[J]. Signal Transduct Target Ther, 2020, 5(1): 229. doi: 10.1038/s41392-020-00323-3
[14] Yamamoto S, Kato K. Immuno-oncology for esophageal cancer[J]. Future Oncol, 2020, 16(32): 2673-2681. doi: 10.2217/fon-2020-0545
[15] Huang J, Xiao JX, Fang WT, et al. Anlotinib in chemotherapy-refractory metastatic esophageal squamous cell carcinoma(ESCC): A randomized, double-blind, multicenter phase Ⅱ trial[J]. J Clin Oncol, 2019, 37(4_suppl): 95. doi: 10.1200/JCO.2019.37.4_suppl.95
[16] Chu L, Chen Y, Liu Q, et al. A Phase Ⅱ Study of Apatinib in Patients with Chemotherapy-Refractory Esophageal Squamous Cell Carcinoma (ESO-Shanghai 11)[J]. Oncologist, 2021, 26(6): e925-e935. doi: 10.1002/onco.13668
[17] Song ZZ, Zhao LF, Zuo J, et al. Clinical Outcomes and Safety of Apatinib Mesylate in the Treatment of Advanced Non-Squamous Non-Small Cell Lung Cancer in Patients Who Progressed After Standard Therapy and Analysis of the KDR Gene Polymorphism[J]. Onco Targets Ther, 2020, 13: 603-613. doi: 10.2147/OTT.S222985
[18] Huang J, Xiao J, Fang W, et al. Anlotinib for previously treated advanced or metastatic esophageal squamous cell carcinoma: A double-blind randomized phase 2 trial[J]. Cancer Med, 2021, 10(5): 1681-1689. doi: 10.1002/cam4.3771
[19] Cheng JD, Chai LX, Zhao ZP, et al. Efficacy and Safety of Anlotinib for Patients with Advanced NSCLC Who Progressed After Standard Regimens and the Preliminary Analysis of an Efficacy Predictor[J]. Cancer Manag Res, 2020, 12: 5641-5650. doi: 10.2147/CMAR.S253366
[20] Li J, Wang L. Efficacy and safety of apatinib treatment for advanced esophageal squamous cell carcinoma[J]. Onco Targets Ther, 2017, 10: 3965-3969. doi: 10.2147/OTT.S132756
[21] Geng N, Ding CM, Liu ZK, et al. Influence of VEGFR2 gene polymorphism on the clinical outcomes of apatinib for patients with chemotherapy-refractory extensive-stage SCLC: a real-world retrospective study[J]. Int J Clin Oncol, 2021, 26(4): 670-683. doi: 10.1007/s10147-020-01849-w
-
期刊类型引用(1)
1. 贾晓君,孟晓红,王娜. 阿帕替尼联合替吉奥二线治疗食管癌的疗效及其对循环肿瘤细胞水平的影响. 肿瘤基础与临床. 2024(04): 408-410 .  百度学术
百度学术
其他类型引用(0)



 下载:
下载: