Correlation Between Dose Volume Parameters and Radiation Pneumonitis in Elderly Patients with Esophageal Cancer After Three-dimensional Conformal Radiotherapy
-
摘要:目的
分析剂量体积参数与老年食管癌患者三维适形放疗后发生≥2级放射性肺炎的相关性。
方法收集并分析250例来自不同医疗中心、接受三维适形放疗的老年食管癌患者资料,对患者临床特征行χ2检验,对剂量体积参数等因素行Logistic单因素及多因素分析,确定与发生≥2级放射性肺炎的独立相关因素,并通过ROC曲线分析独立相关因素的最佳分界值。
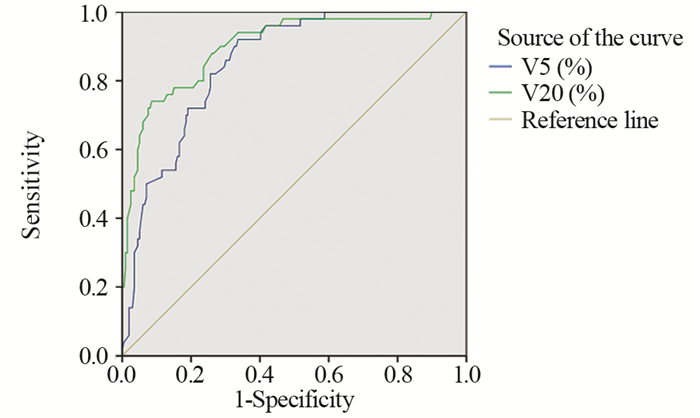
结果(1)三维适形放疗后共20%的老年食管癌患者发生了≥2级放射性肺炎,单因素分析提示双肺V5、V10、V20、V30及肺平均剂量(MLD)是≥2级放射性肺炎的相关因素;(2)多因素分析提示双肺V5、V20是≥2级放射性肺炎的独立相关因素;(3)ROC曲线提示控制放射性肺炎发生的最佳分界值为V5<53.9%,V20<23.2%。
结论双肺V5和V20是老年食管癌患者三维适形放疗后发生≥2级放射性肺炎的独立相关因素。
Abstract:ObjectiveTo analyze the correlation between ≥grade 2 radiation pneumonitis (RP) and dose volume parameters of elderly patients with esophageal cancer after three-dimensional conformal radiotherapy.
MethodsWe collected the data of 250 elderly patients with esophageal cancer who received three-dimensional conformal radiotherapy from different medical centers. The clinical features of patients were analyzed by Chi-square test while dose volume parameters were analyzed by Logistic univariate and multivariate analyses. ROC curve was used to determine the best cut-off value.
ResultsAfter three-dimensional conformal radiotherapy, 20% of patients developed ≥grade 2 RP. Univariate analysis showed that bilateral pulmonary V5, V10, V20, V30 and mean lung dose were associated with ≥grade 2 RP but multivariate analysis revealed that only V5 and V20 were independent relevant factors of RP. ROC curve indicated that the best cut-off value was V5 < 52.9% and V20 < 23.2%.
ConclusionBilateral pulmonary V5 and V20 are independently related to ≥grade 2 RP in elderly patients with esophageal cancer after 3-D conformal radiotherapy.
-
0 引言
子宫内膜癌是在北美和欧洲最常见的女性恶性肿瘤,近年来我国的发病率明显增加,发病人群年轻化[1]。子宫内膜癌的发病机制目前尚未完全明确,随着研究的深入,越来越清晰地认识到子宫内膜癌是多种因素协同交叉作用、具有不同遗传和分子特征的一类疾病[2-3]。根据肿瘤发病机制与雌激素依赖相关性,将其分为Ⅰ型和Ⅱ型,80%以上为Ⅰ型子宫内膜癌。遗传易感性是导致个体对相同致癌因素敏感度不一致的重要因素,某些雌激素代谢通路中关键酶可以改变体内雌激素或外源性雌激素及其代谢产物的水平。基因多态性与酶活性有关,雌激素代谢酶基因多态性可能导致子宫内膜癌发病易感性差异[4]。本研究利用SNP分型检测技术,探索雌激素代谢关键酶CYP1B1、CYP1A1和NQO1基因的单核苷酸多态性(single nucleotide polymorphisms, SNPs)位点分布频率与Ⅰ型子宫内膜癌易感性之间关系,以期对Ⅰ型子宫内膜癌的易感人群进行筛查。
1 资料与方法
1.1 临床资料
抽取我院2014年3月—2016年10月收治的经病理诊断为子宫内膜样腺癌的103例患者和同期子宫内膜正常的100例其他疾病患者静脉外周全血2~3 ml,作为检测标本。病例和对照组均取得患者的知情同意并详细询问病史,包括初潮年龄、生育状况、绝经年龄、孕产次数、内科并发症及肿瘤家族史。测量身高与体重,并计算体质指数(body mass index, BMI),测量血压,采集静脉血化验空腹血糖(FPG)及血脂、肿瘤标志物[5-6]。年龄31~78岁,平均(51.15±9.69)岁,子宫内膜癌组平均年龄(54.77±8.21)岁,对照组为(46.95±9.65)岁。
1.2 方法
1.2.1 确定候选基因及其SNPs位点
由于Ⅰ型子宫内膜癌为雌激素依赖性肿瘤,结合相关文献报道,在美国国家生物技术信息中心(National Center for Biotechnology Information, NCBI)人类基因组数据库中选择与雌激素代谢相关的关键酶CYP1A1、CYP1B1,具有抗癌突变的依赖还原型辅酶Ⅰ/Ⅱ醌氧化还原酶1(quinone oxido-reductase1, NQO1)3个基因,选取与代谢酶活性密切相关且杂合度大于10%的SNPs位点进行基因型分析。
1.2.2 引物设计合成
利用引物设计软件premier 5.0,在含SNPs的DNA序列上设计PCR引物,并将设计的引物进行引物的同源性比较,选出同源性最小而Tm值和G/C比值合适的引物作为PCR反应引物,所扩增片段应包含要检测的SNPs位点,其位置尽可能设计在PCR扩增片段的中部。
1.2.3 检测方法
使用DNA提取试剂盒(DP304-03)从外周血中提取DNA,进行预扩增,琼脂糖凝胶电泳检测DNA完整性。根据设计引物模板,进行延伸反应及延伸产物纯化。PCR扩增反应体系总体积为50 μl,内含5 μl 10×PCR缓冲液,3 μl 25 mmol/L MgCl2溶液,5 μl 2 mmol/L dNTP混合物,1.25 U Taq DNA聚合酶,0.5 μmol/L引物及100g的基因组DNA。反应在MJ公司PTC-100 PCR反应仪中进行。PCR循环条件为95℃预变性10 min,然后94℃变性30 s,60℃复性30 s,72℃延伸30 s,反应40个循环后,72℃再延伸10 min。扩增产物用2%的琼脂糖凝胶电泳检测,4℃保存。PCR产物经纯化后作为测序模板,用Big-Dye末端荧光标记试剂盒进行测序反应,测序反应产物经纯化后在ABI公司3730XL测序仪上进行测序电泳,用Gene codes公司的Sequencer4.2对测序结果进行分析。
1.3 统计学方法
采用SPSS18.0软件对实验数据进行统计学分析,用χ2检验和多因素Logistic回归模型分析各基因型在两组人群中的分布差异及其与子宫内膜癌临床病理特征的相关性,P < 0.05为差异有统计学意义。
2 结果
2.1 多因素回归分析
CYP1B1基因SNPs位点rs111888224因无碱基改变未纳入统计,rs1056836、rs2551188、rs10916在研究人群中均存在多态性,但分布频率差异无统计学意义;rs1056836 C、G基因分布频率差异有统计学意义(P=0.0454)。
CYP1A1基因SNP位点rs4646421在研究人群中存在CT、CC、TT多态性。与CT型相比,CC型为保护基因型,OR=0.479(95%CI: 0.255~0.899),差异有统计学意义(P=0.0219),其C、T基因分布频率差异有统计学意义(P=0.0041),见表 1。
表 1 基因型/等位基因在Ⅰ型子宫内膜癌患者中的分布(n(%))Table 1 Genotype/alleles distributions in typeⅠendometrial carcinoma cases (n(%))
2.2 CYP1A1基因rs4646421位点多态性与Ⅰ型子宫内膜癌危险因素的分层分析
对CYP1A1基因rs4646421位点不同基因型与Ⅰ型子宫内膜癌的危险因素相关性进行分层比较,发现在年龄 > 60岁、BMI≥25、绝经延迟、并发高血压的个体中,携带CT+TT突变基因型将增加罹患Ⅰ型子宫内膜癌的风险(P < 0.05),见表 2。
表 2 发病危险因素与基因型分布的关系(n(%))Table 2 Association between risk factors and genotypes distributions of SNP(rs4646421) in CYP1A1 (n(%))
3 讨论
雌激素分布在细胞内外表面,通过雌激素受体的核内结合蛋白在靶细胞中保持高亲和力和特异性。涉及雌激素生物合成和代谢的基因的多态性是雌激素受体阳性恶性肿瘤的潜在危险因素,有几种细胞色素P450(CYP)酶参与雌激素的氧化代谢途径[7]。CYP1A1和CYP1B1是代谢产生2-羟基-4-羟基雌激素代谢物的基本酶[8-9],在不同人群中不仅存在多态性,并且在性激素相关组织中的表达高于细胞色素P450超家族的其他成员,其基因多态性与乳腺癌、子宫肌瘤、子宫内膜异位症、宫颈癌等疾病风险相关[10-14]。
CYP1A1基因定位于人类染色体15q22-24,编码由512个氨基酸组成的蛋白质,是细胞色素P450家族中高诱导成员。CYP1A1酶的激活参与内源性和外源性化合物的氧化作用,催化多环芳香族碳氢化合物转化为酚与环氧化合物。一些酚类和环氧化合物可与DNA结合形成加合物,最终转化为致癌物、二醇环氧化物,通过环氧化物水解酶与一些抗肿瘤药物发生耐药,并增加个体肿瘤风险[9, 15]。本研究结果显示CYP1A1基因SNP位点rs4646421在子宫内膜癌人群中存在CT、CC、TT多态性,与携带CT基因型相比,携带CC基因型的个体罹患子宫内膜癌的风险较小,携带T等位基因的个体子宫内膜癌的发病风险高于C等位基因携带者。与携带CC基因型相比,携带TC+TT基因型在年龄 > 60岁、BMI≥25、绝经延迟(超过52岁)、合并高血压的女性中Ⅰ型子宫内膜癌的发病风险增加。
CYP1B1基因位于染色体2q21-22,其单核苷酸多态性SNP总数有353个,目前研究较多的是SNPrs1056836,其mRNA发生1294位碱基胞嘧啶(cytosine, C)变异为鸟嘌呤(guanine, G),使DNA两条链形成野生CC型,杂合CG型和变异GG型3种基因型,即基因多态性。这种改变导致第三外显子432密码子的碱基由CTG变异为GTG,由其编码的亮氨酸(leucine, Leu)变异为缬氨酸(valine, Val),导致CYP1B1酶蛋白功能的改变[16]。CYP1B1通过多方面影响肿瘤的易感性和预后,在胰岛素抵抗中产生作用,突变型表达的酶之蛋白质表现出更强的激活癌前物质活性,或影响睾酮的6β-羟化作用及性激素的激活代谢等[17-18]。
本研究中CYP1B1基因SNP rs2551188、rs10916两个位点在人群中存在多态性,但分布频率无统计学差异;rs1056836在正常子宫内膜组与子宫内膜癌两组间的基因型分布频率无差异,但发生更多的碱基胞嘧啶变异为鸟嘌呤,可能会使罹患子宫内膜癌的风险增加,以往文献中发现对于单个位点基因突变的表现为阴性结论时,多个位点联合的单倍体基因型研究却往往能得到阳性结论,似乎预示着CYP1B1基因在分子遗传学上有一定的倾向性[16-19],但其与肿瘤易感性及预后的机制问题还存在着诸多争议与疑惑,有待于进一步研究。
NQO1是一种重要的化学致癌物质代谢酶,具有抗癌作用,可使内源性及外源性醌类化合物通过电子还原反应变成低毒性的氢醌类物质,再转化为水溶性化合物排出体外,降低了细胞发生突变及癌变的概率。如果位点发生突变,使原位编码的氨基酸发生改变,将会导致该酶活性减弱或完全丧失,降低解毒功能且不能维护细胞稳定,增加某些易感个体细胞发生癌变[20]。报道显示NQO1蛋白高表达在卵巢癌、乳腺癌、结直肠癌等肿瘤中,与临床分期晚、分化程度差和淋巴结转移等临床特征有关[21-22],本研究选择的SNP位点rs45488899和rs1800566虽在两组间均存在基因多态性,但分布频率不具有差异性,因此认为这两个SNP位点与Ⅰ型子宫内膜癌的发生无显著相关性。
本研究通过基因表达谱差异的分析,推测CYP1A1基因rs4646421位点的多态性在Ⅰ型子宫内膜癌的发生发展中起着重要作用,可作为进行高危人群筛查的基因位点,还可以突变的基因型为基础,采取针对性的基因靶向治疗,或者通过修正基因突变谱来改善其预后。但是,基因多态性受复杂的遗传、环境因素、种族等的影响,并与样本量的大小有关,必须经过大样本试验来进一步明确基因多态性与子宫内膜癌易感性之间的基因环境交互作用及具体机制。
Competing interests: The authors declare that they have no competing interests.作者贡献:汪盛:设计及实施研究、采集整理数据、统计分析及撰写论文王彩莲:论文选题、修改论文范丽华:采集整理数据 -
表 1 250例老年食管癌患者的临床特征及卡方检验结果
Table 1 Clinical features and chi-square test results of 250 elderly patients with esophageal cancer

表 2 250例老年食管癌患者发生≥2级RP的Logistic单因素分析
Table 2 Logistic univariate analysis for ≥grade 2 RP in 250 elderly patients with esophageal cancer

表 3 250例老年食管癌患者发生≥2级RP的Logistic多因素分析
Table 3 Logistic multivariate analysis for ≥grade 2 RP in 250 elderly patients with esophageal cancer

表 4 双肺V5和V20的ROC曲线下面积
Table 4 Area under ROC curve of bilateral pulmonary V5 and V20

表 5 双肺V5和V20的最佳分界值
Table 5 The best cut-off value of bilateral pulmonary V5 and V20

-
[1] Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68: 394-424. doi: 10.3322/caac.21492
[2] 陈茹, 郑荣寿, 张思维, 等. 2015年中国食管癌发病和死亡情况分析[J]. 中华预防医学杂志, 2019, 53(11): 1094-1097. https://www.cnki.com.cn/Article/CJFDTOTAL-ZHLU201204001.htm Chen R, Zheng RS, Zhang SW, et al. Analysis of incidence and mortality of esophageal cancer in China, 2015[J]. Zhonghua Yu Fang Yi Xue Za Zhi, 2019, 53(11): 1094-1097. https://www.cnki.com.cn/Article/CJFDTOTAL-ZHLU201204001.htm
[3] Castillo R, Pham N, Castillo E, et al. Pre-radiation therapy fluorine 18 fluorodeoxyglucose PET helps identify patients with esophageal cancer at high risk for radiation pneumonitis[J]. Radiology, 2015, 275(3): 822-831. doi: 10.1148/radiol.14140457
[4] 马晓洁, 胡劲, 谭榜宪. 老年食管癌三维适形放疗致急性放射性肺炎的临床危险因素[J]. 中国老年学杂志, 2015, 35(2): 486-488. https://www.cnki.com.cn/Article/CJFDTOTAL-ZLXZ201502096.htm Ma XJ, Hu J, Tan BX. Clinical risk factors of acute radiation pneumonia caused by three-dimensional conformal radiotherapy for esophageal cancer in the elderly patients[J]. Zhongguo Lao Nian Xue Za Zhi, 2015, 35(2): 486-488. https://www.cnki.com.cn/Article/CJFDTOTAL-ZLXZ201502096.htm
[5] Lu C, Lei Z, Wu HB, et al. Evaluating risk factors of radiation pneumonitis after stereotactic body radiation therapy in lung tumor: meta-analysis of 9 observational studies[J]. PLoS One, 2018, 13(12): e0208637. doi: 10.1371/journal.pone.0208637
[6] Tsujino K, Tekatli H, Shimada T, et al. Combinedanalysis of V20, VS5, pulmonary fibrosis score on baseline computed tomography, and patient age improves prediction of severe radiation pneumonitis after concurrent chemoradiotherapy for locally advanced nonsmall-cell lung cancer[J]. J Thorac Oncol, 2014, 9: 983-990. doi: 10.1097/JTO.0000000000000187
[7] Boyle J, Ackerson B, Gu L, et al. Dosimetric advantages of intensity modulated radiation therapy in locally advanced lung cancer[J]. Adv Radiat Oncol, 2017, 2(1): 6-11. doi: 10.1016/j.adro.2016.12.006
[8] Tekatli H, Duijm M, Oomen-de Hoop E, et al. Normal tissue complication probability modeling of pulmonary toxicity after stereotactic and hypofractionated radiation therapy for central lung tumors[J]. Int J Radiation Oncol Biol Phys, 2018, 100(3): 738-747. doi: 10.1016/j.ijrobp.2017.11.022
[9] 李晔雄. 肿瘤放射治疗学[M]. 5版. 北京: 中国协和医科大学出版社, 2018: 790. Li YX. Radiation Oncology[M]. 5th. Edition. Beijing: Peking Union Medical College Press, 2018: 790.
[10] National Cancer Institute (NIH). Common Terminology Criteria for Adverse Events (CTCAE)[EB/OL]. (2018-01-25). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50.
[11] Huang Y, Zhang W, Yu F, et al. The cellular and molecular mechanism of radiation-induced lung injury[J]. Med Sci Monit, 2017, 23: 3446-3450. doi: 10.12659/MSM.902353
[12] Yorke ED, Jackson A, Rosenzsweig KE, et al. Dose-volume factors contributing to the incidence of radiation pneumonitis in non-small cell lung cancer patients treated with three-dimensional conformal radiation therapy[J]. Int J Radiat Oncol Biol Phys, 2002, 54(2): 329-339. http://onlinelibrary.wiley.com/resolve/reference/PMED?id=12243805
[13] Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung[J]. Int J Radiat Oncol Biol Phys, 2010, 76(3 Suppl): S70-S76. http://www.zhangqiaokeyan.com/academic-journal-foreign-pmc_detail_thesis/040005945408.html
[14] Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: An international individual patient data meta-analysis[J]. Int J Radiat Oncol Biol Phys, 2013, 85(2): 444-450. doi: 10.1016/j.ijrobp.2012.04.043
[15] Roman MA, Rossiter HB, Casaburi R. Exercise, ageing and the lung[J]. Eur Respir J, 2016, 48(5): 1471-1486. doi: 10.1183/13993003.00347-2016
[16] Deng G, Liang N, Xie J, et al. Pulmonary toxicity generated from radiotherapeutic treatment of thoracic malignancies[J]. Oncol Lett, 2017, 14(1): 501-511. doi: 10.3892/ol.2017.6268
[17] Graves PR, Siddiqui F, Anscher MS, et al. Radiation pulmonary toxicity: from mechanisms to management[J]. Semin Radiat Oncol, 2010, 20(3): 201-207. doi: 10.1016/j.semradonc.2010.01.010
[18] Liang B, Tian Y, Chen X, et al. Prediction of radiation pneumonitis with dose distribution: a convolutional neural network (CNN) based model[J]. Front Oncol, 2020, 9: 1500. doi: 10.3389/fonc.2019.01500
[19] Cui Z, Tian Y, He B, et al. Associated factors of radiation pneumonitis induced by precise radiotherapy in 186 elderly patients with esophageal cancer[J]. Int J Clin Exp Med, 2015, 8(9): 16646-16651. http://europepmc.org/articles/PMC4659085/
[20] Kong M, Lim YJ, Kim Y, et al. Diabetes mellitus is a predictive factor for radiation pneumonitis after thoracic radiotherapy in patients with lung cancer[J]. Cancer Manag Res, 2019, 11: 7103-7110. doi: 10.2147/CMAR.S210095
[21] Wen G, Tan YT, Lan XW, et al. New clinical features and dosimetric predictor identification for symptomatic radiation pneumonitis after tangential irradiation in breast cancer patients[J]. J Cancer, 2017, 8(18): 3795-3802. doi: 10.7150/jca.21158
[22] Tonison JJ, Fischer SG, Viehrig M, et al. Radiation pneumonitis after intensity-modulated radiotherapy for esophageal cancer: institutional data and a systematic review[J]. Sci Rep, 2019, 9(1): 2255. doi: 10.1038/s41598-018-38414-5
[23] Skloot GS. The effects of aging on lung structure and function[J]. Clin Geriatr Med, 2017, 33(4): 447-457. doi: 10.1016/j.cger.2017.06.001
[24] Lan K, Zhu J, Zhang J, et al. Propensity score-based comparison of survival and radiation pneumonitis after definitive chemoradiation for esophageal cancer: Intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy[J]. Radiother Oncol, 2020, 149: 228-235. doi: 10.1016/j.radonc.2020.05.036
[25] Kumar G, Rawat S, Puri A, et al. Analysis of dose-volume parameters predicting radiation pneumonitis in patients with esophageal cancer treated with 3D-conformal radiation therapy or IMRT[J]. Jpn J Radiol, 2012, 30(1): 18-24. doi: 10.1007/s11604-011-0002-2
[26] Lin JB, Hung LC, Cheng CY, et al. Prognostic significance of lung radiation dose in patients with esophageal cancer treated with neoadjuvant chemoradiotherapy[J]. Radiat Oncol, 2019, 14(1): 85. doi: 10.1186/s13014-019-1283-3
[27] 沈文斌, 祝淑钗, 高红梅, 等. 肺脏低剂量区体积预测食管癌三维适形放疗所致急性放射性肺炎的价值[J]. 中华肿瘤杂志, 2013, 35(1): 45-49. https://www.cnki.net/KCMS/detail/detail.aspx?dbcode=IPFD&filename=ZGKA201205001195&dbname=IPFD9914 Shen WB, Zhu SC, Gao HM, et al. Low dose volume histogram analysis of the lungs in prediction of acute radiation pneumonitis in patients with esophageal cancer treated with three-dimensional conformal radiotherapy[J]. Zhonghua Zhong Liu Za Zhi, 2013, 35(1): 45-49. https://www.cnki.net/KCMS/detail/detail.aspx?dbcode=IPFD&filename=ZGKA201205001195&dbname=IPFD9914
[28] Zhao Y, Chen L, Zhang S, et al. Predictive factors for acute radiation pneumonitis in postoperative intensity modulated radiation therapy and volumetric modulated arc therapy of esophageal cancer[J]. Thorac Cancer, 2015, 6(1): 49-57. doi: 10.1111/1759-7714.12142
[29] 杜峰, 王强, 王玮, 等. 胸段食管癌放疗后放射性肺炎相关因素分析[J]. 中华放射医学与防护杂志, 2020, 40(11): 832-839. Du F, Wang Q, Wang W, et al. Analysis of related factors of radiation pneumonitis after radiotherapy for thoracic segment esophageal cancer[J]. Zhonghua Fang She Yi Xue Yu Fang Hu Za Zhi, 2020, 40(11): 832-839.
[30] 姚波, 王雅棣, 刘清志, 等. 肺癌与食管癌螺旋断层放疗致放射性肺炎的临床观察[J]. 临床肿瘤学杂志, 2014, 19(1): 52-56. https://www.cnki.com.cn/Article/CJFDTOTAL-LCZL201401012.htm Yao B, Wang YL, Liu QZ, et al. Radiation pneumonitis in patients with lung cancer and esophageal cancer treated by helical tomotherapy[J]. Lin Chuang Zhong Liu Xue Za Zhi, 2014, 19(1): 52-56. https://www.cnki.com.cn/Article/CJFDTOTAL-LCZL201401012.htm



 下载:
下载: