Effects of Astragalus Polysaccharides on Growth, Metastasis and Cell Cycle of Lewis Lung Cancer in Tumor-bearing Mice with Qi and Yin Deficiency
-
摘要:目的
观察黄芪多糖抑制气阴两虚Lewis肺癌荷瘤小鼠的生长、转移及对肺癌细胞周期的影响。
方法体外培养Lewis肺癌细胞,随机分为对照组和中药组,流式细胞术检测细胞周期;C57BL/6J小鼠90只,设空白组10只,余80只刨花烟熏并灌胃温热性的中药,移植Lewis肺癌实体肿瘤建立气阴两虚荷瘤小鼠模型并随机分为8组,比较各组小鼠抑瘤率及q值,计数外周血细胞及骨髓细胞,ELISA测定血清IL-2、IFN-γ、TNF-α、IL-10、HIF-1α、VEGF、MMP-2的含量。
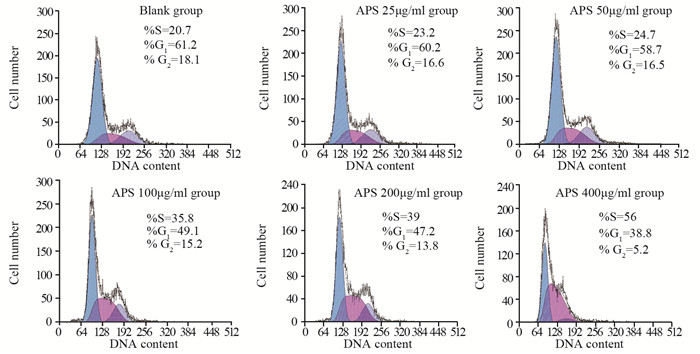
结果黄芪多糖将Lewis肺癌细胞阻滞于S期,随着药物浓度的增加,细胞凋亡逐渐增加;联合用药各组的抑瘤率高于黄芪多糖各组和顺铂组,q值均在0.85~1.15之间;与顺铂组比较,联合用药中、高剂量组小鼠外周血细胞及骨髓细胞的数量,IL-2、INF-γ、TNF-α均显著升高,IL-10、HIF-1α、VEGF、MMP-2均显著降低(P < 0.05, P < 0.01)。
结论黄芪多糖在体外对Lewis肺癌细胞有抑制作用,体内与顺铂联合能抑制气阴两虚Lewis荷瘤小鼠肺癌细胞的生长、转移,提高外周血细胞和骨髓细胞的细胞数量,提高IL-2、INF-γ、TNF-α的含量,减少IL-10、HIF-1α、VEGF、MMP-2的含量。
Abstract:ObjectiveTo observe the effects of Astragalus polysaccharides on the growth, metastasis and cell cycle of Lewis lung cancer in tumor-bearing mice with Qi and Yin deficiency.
MethodsLewis lung cancer cells cultured in vitro were randomly divided into control group and Chinese medicine group. Cell cycle was detected by flow cytometry. Among 90 C57BL/6J mice, 10 mice were taken as blank group, and the other 80 mice were treated with Chinese medicine and gastric perfusion of warm smoked shavings. The mice bearing transplanted Lewis lung cancer solid tumor with Qi and Yin deficiency were randomly divided into eight groups. Mice tumor inhibition rate and q value were compared. Peripheral blood cells and bone marrow cells were counted and ELISA was applied to detect the content of serum IL-2, IFN-γ, TNF-α, IL-10, HIF-1α, VEGF and MMP-2.
ResultsAstragalus polysaccharide arrested Lewis lung cancer cells in S phase; with the increase of drug concentration, cell apoptosis increased gradually; the tumor inhibition rates in the drug combination groups were higher than those of Astragalus polysaccharide groups and cisplatin group, q value was 0.85-1.15; compared with cisplatin group, the number of peripheral blood cells and bone marrow cells, the content of IL-2, INF-γ and TNF-α in moderate-and high-dose drug combination groups were significantly increased, while the content of IL-10, HIF-1α, VEGF and MMP-2 were significantly decreased (P < 0.05, P < 0.01).
ConclusionAstragalus polysaccharides in vitro has inhibitory effect on Lewis lung cancer cells; its combination with cisplatin in vivo could inhibit the growth and metastasis of Lewis lung cancer cells, increase the number of peripheral blood cells and bone marrow cells, the content of IL-2, INF-γ and TNF-α, and reduce the content of IL-10, HIF-1α, VEGF and MMP-2 in tumor-bearing mice with Qi and Yin deficiency.
-
Key words:
- Astragalus polysaccharide /
- Qi and Yin deficiency /
- Lewis lung cancer /
- Cell cycle /
- Antitumor effect
-
0 引言
鼻咽癌是最常见的头颈部肿瘤之一。我国为鼻咽癌高发地区,每年的发病率约为20/10万[1],由于鼻咽解剖结构及生物学行为的特殊性,很难行手术治疗,目前鼻咽癌公认和有效的治疗手段为放射治疗或以放疗为主的综合治疗。虽然放疗技术不断进步与放疗设备的不断更新,鼻咽癌的生存率有了较大的提高,但5年生存率仍为60%~80%[2],部分患者仍未能获得长期生存。TNM分期系统是鼻咽癌预后判断和指导治疗的重要依据,但临床发现同一分期患者即使接受相同的治疗方案,预后却不同[3-4],这提示鼻咽癌生物学差异的存在,仅基于解剖学信息的TNM临床分期系统还不能准确地预测鼻咽癌患者的预后。虽然EB病毒滴度、表皮生长因子受体、microRNA也可提示鼻咽癌的预后[5-7],但检测成本高,需要多中心合作,临床上可行性差。所以,亟需检测方便、价格低廉可预测鼻咽癌预后的标志物。
流行病学研究证实,约25%的肿瘤由炎性反应发展而来,其与肿瘤的发生发展密切相关并且影响肿瘤患者的预后[8]。炎性反应指标,如白细胞计数[9]、血小板计数[10-11]、中性粒淋巴细胞比(neutrophil-lymphocyte ratio, NLR)[12-13]、血小板淋巴细胞比(platelet-lymphocyte ratio, PLR)[14-15]被发现可作为肿瘤的独立预后因素。这些血液指标检测方便,价格低廉,可广泛应用于临床,评估患者预后。本研究通过对91例鼻咽癌患者临床资料进行回顾性分析,评价治疗前PLR和NLR与鼻咽癌患者预后的相关性,为评估预后提供客观依据。
1 资料与方法
1.1 临床资料
回顾性收集2009年1月至2013年9月期间于西安交通大学第一附属医院和陕西省人民医院初治的91例鼻咽癌患者,所有病例均经病理证实。临床资料完整。排除标准:(1)合并有免疫性疾病以及其他恶性肿瘤的患者;(2)治疗前合并有急性或慢性感染;(3)合并有血液系统疾病、血栓或出血性疾病;(4)合并有严重的肝、肾疾病;(5)治疗前曾接受过放疗或化疗;(6)无远处转移。记录患者治疗前的中性粒细胞计数、淋巴细胞计数及血小板计数结果。
1.2 治疗及随访方法
入选患者采用3D-CRT或IMRT根治性放疗(有或无化疗),Ⅰ期患者仅接受单纯放射治疗,Ⅱ、Ⅲ、Ⅳ期患者接受以顺铂和5-氟尿嘧啶为主的辅助或同步放化疗。鼻咽原发灶和颈部转移淋巴结剂量为(70~76)Gy/(7~8)w/(35~38)f,颈部预防区域剂量为(50~60)Gy/(5~6)w/(25~30)f。根据患者的临床分期及不良反应给予2~6周期的全身化疗,化疗方案为:顺铂25 mg/m2,第1~3天静脉滴注;5-氟尿嘧啶500 mg/m2,第1~5天静脉滴注,每21天重复1周期。患者治疗结束后均定期随访,治疗后前2年,每3月检查一次,2年后半年复查一次,5年后1年复查1次。随访截止时间为2016年9月。
1.3 统计学方法
采用SPSS19.0软件对数据进行统计学分析。绘制ROC曲线确定PLR和NLR与总生存期(overall survival, OS)及无进展生存期(progression-free survival, PFS)的相关性,选取截断值。应用Kaplan-Meier法进行生存分析并采用Log rank检验来检验。采用Cox比例风险回归模型分析多种因素对生存时间的影响。以P < 0.05为差异有统计学意义。
2 结果
2.1 鼻咽癌患者临床病理资料
91例患者的基本特征资料见表 1,中位年龄53岁(12~72)岁,女30例,男61例,男女比例2:1,Ⅰ、Ⅱ、Ⅲ、Ⅳ期患者分别为2、27、42、20例。单纯放疗患者9例,82例患者接受辅助或同步放化疗,所有患者均按期完成放化疗。中位随访时间为44月(6~87)月,其中44例出现复发或转移,39例患者死亡。患者的1、3、5年总生存率分别为92.3%、72.1%、56.8%,1、3、5年无进展生存率分别为82.4%、60.9%、53.3%。
表 1 91例鼻咽癌患者临床基本特征资料(n(%))Table 1 Basic clinical features of 91 nasopharyngeal carcinoma patients (n(%))
2.2 ROC曲线选取PLR和NLR预后相关截断值
以OS作为终点,PLR、NLR为检测变量,绘制ROC曲线选取截断值分别为143.3、2.6,两者的曲线下面积分别为0.640、0.739,见图 1。
以PFS作为终点,PLR、NLR为检测变量,绘制ROC曲线选取截断值分别为143.3、2.6,两者的曲线下面积分别为0.657、0.694,见图 2。说明治疗前PLR、NLR与患者的预后存在相关性,利用ROC曲线选取的截断值进行进一步生存分析。
2.3 Kaplan-Meier生存分析、Cox单因素和多因素分析
PLR≥143.3组和PLR < 143.3组患者生存曲线比较,差异有统计学意义(P=0.022),见图 3~4。NLR≥2.6组和NLR < 2.6组患者生存曲线比较,差异有统计学意义(P=0.044),见图 5~6。
Cox单因素分析显示除性别、年龄以外,TNM分期、治疗前PLR≥143.3、NLR≥2.6均是影响鼻咽癌患者OS和PFS的不良预后因素(P < 0.05),见表 2。Cox多因素分析显示治疗前PLR≥143.3(RR=2.491, 95%CI=1.139~5.451, P=0.022)、NLR≥2.6(RR=2.186, 95%CI=1.021~4.682,P=0.044)是鼻咽癌患者OS的独立危险因素,而治疗前PLR≥143.3(RR=2.461,95%CI=1.242~4.874, P=0.010)是鼻咽癌患者PFS的独立危险因素,见表 3。
表 2 影响鼻咽癌患者生存预后的Cox单因素分析Table 2 Cox univariate analysis of prognostic factors for nasopharyngeal carcinoma patients 表 3 影响鼻咽癌患者生存预后的Cox多因素分析Table 3 Cox multivariate analysis of prognostic factors for nasopharyngeal carcinoma patients
表 3 影响鼻咽癌患者生存预后的Cox多因素分析Table 3 Cox multivariate analysis of prognostic factors for nasopharyngeal carcinoma patients
3 讨论
鼻咽癌对放射线高度敏感,因此放疗成为主要治疗手段。随着三维适形放疗和调强放射治疗的临床应用,鼻咽癌的生存率较前明显提高,但5年生存率仍仅为60%~80%。多项研究表明鼻咽癌患者预后与众多因素有关,包括患者年龄、临床分期、EB病毒感染及贫血等。此外,肿瘤的预后还与机体本身的炎性反应有关。炎性反应包含中性粒细胞、淋巴细胞、血小板、C反应蛋白等多种指标,其中PLR、NLR已受到越来越多专家的关注。本研究发现治疗前PLR和NLR可能成为鼻咽癌的独立预后因素。
恶性肿瘤患者常伴随血小板的升高,实验研究表明血小板参与肿瘤细胞生长、转移及血管生成[16]。临床研究表明血小板数目升高与肿瘤患者较差预后相关[11, 17]。此外研究表明中性粒细胞可促使机体产生多种促肿瘤生长因子和蛋白酶,促进肿瘤的发生、发展[18]。而淋巴细胞参与机体的免疫反应是抗肿瘤免疫的重要组成部分,淋巴细胞减少说明机体免疫机制异常,抗肿瘤免疫力下降,为肿瘤生长、浸润和转移提供条件。随着肿瘤进展,机体内炎性反应与肿瘤失去平衡,体内淋巴细胞降低,而血小板、中性粒细胞升高,相应的PLR和NLR比值也增高,机体内促进肿瘤炎性反应与抗肿瘤炎性反应的平衡状态被打破。因此PLR和NLR是反应机体免疫情况的重要指标,两者的升高能促进肿瘤进展,导致肿瘤患者预后不良。既往研究结果显示高PLR和NLR可影响宫颈癌、乳腺癌、结直肠癌等恶性肿瘤的预后[19-21]。而目前关于PLR、NLR与鼻咽癌患者预后相关性的研究较少,Sun等[21]分析了251例鼻咽癌患者治疗前PLR和NLR,结果证明治疗前两者水平是影响鼻咽癌患者生存独立预后因素。本研究结果显示治疗前PLR、NLR与鼻咽癌患者的总生存期和无进展生存期具有相关性。Cox多因素分析提示PLR≥143.3、NLR≥2.6和TNM分期是影响鼻咽癌患者治疗后的独立危险因素。PLR≥143.3组患者有较短OS和PFS,而NLR≥2.6组患者有较差的OS,和本研究结果相一致。因此,高PLR、NLR的鼻咽癌患者总生存率要低于低PLR、NLR的患者,且高PLR的患者复发或转移风险明显增加。据此,临床上或许可以通过提高鼻咽癌患者免疫功能及降低机体炎性反应,改善患者的预后。
但由于本研究是一个相对小样本的回顾性研究,不能代表大部分的鼻咽癌患者,且随访时间较短,存在一定的局限性,因此需要进行多中心、大样本的前瞻性研究来进一步证实。
本研究结果表明,治疗前PLR和NLR水平与鼻咽癌患者预后具有相关性,可能是影响鼻咽癌患者预后的独立危险因素,NLR和PLR的获取具有简便、经济的优点,可以作为鼻咽癌患者病情评估的一个有益补充,值得推广。目前鼻咽癌相关有效预后指标较多,笔者将在今后的临床研究工作中继续探索,将本研究指标与已有的有效预后指标进行比较,从而提高治疗前PLR和NLR水平这一预后指标应用于临床的合理性及可靠性。
-
表 1 黄芪多糖对Lewis肺癌细胞周期分布的影响(%, x±s, n=3)
Table 1 Influence of astragalus polysaccharide(APS) on periodic distribution of Lewis lung cancer cell(LLC) (%, x±s, n=3)

表 2 各组小鼠平均瘤体积、瘤重、抑瘤率(x±s, n=10)
Table 2 Average tumor volume, tumor weight and tumor inhibitory rate of mice in each group (x±s, n=10)

表 3 各组小鼠骨髓细胞及外周血细胞数比较(x±s, n=10)
Table 3 Comparison of bone marrow cells number and peripheral blood cells number among each group of mice (x±s, n=10)

表 4 各组小鼠血清IL-2、INF-γ、TNF-α和IL-10含量的比较(x±s, n=10)
Table 4 Contents comparison of serum IL-2, INF-γ, TNF-α and IL-10 among each group of mice (x±s, n=10)

表 5 各组小鼠血清HIF-1α、VEGF和MMP-2水平的比较(x±s, n=10)
Table 5 Comparison of serum HIF-1α, VEGF, MMP-2 levels among each group of mice (x±s, n=10)

-
[1] Jemal A, Bray F, Center MM, et al. Global cancer statistics[J]. CA Cancer J Clin, 2011, 61(2): 69-90. doi: 10.3322/caac.v61:2
[2] 白崇智, 仲启明, 武玉鹏, 等.黄芪等5种中药对小鼠辐射损伤防护作用的实验研究[J].细胞与分子免疫学杂志, 2013, 29(10): 1052-4. http://med.wanfangdata.com.cn/Paper/Detail/PeriodicalPaper_xbyfzmyxzz201310013 Bai CZ, Zhong QM, Wu YP, et al. Experimental study on the protective effects of astragalus root and other 5 kinds of traditional Chinese medicine on radiation injury in mice[J]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi, 2013, 29(10): 1052-4. http://med.wanfangdata.com.cn/Paper/Detail/PeriodicalPaper_xbyfzmyxzz201310013
[3] 刘跃华, 黄静, 王雍, 等.注射用黄芩多糖联合化疗治疗中晚期胃癌的疗效[J].实用医学杂志, 2011, 27(3): 516-8. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=syyxzz201103067 Liu YH, Huang J, Wang Y. Efficacy of combined chemotherapy with Radix and Radix polysaccharides in the treatment of advanced gastric cancer[J]. Shi Yong Yi Xue Za Zhi, 2011, 27(3): 516-8. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=syyxzz201103067
[4] 明海霞, 陈彦文, 张帆, 等.黄芪多糖联合顺铂处理降低Lewis肺癌移植瘤CD44表达并降低血清Ⅳ型胶原蛋白和透明质酸的水平[J].细胞与分子免疫学杂志, 2015, 31(7): 909-13. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xbyfzmyxzz201507010 Ming HX, Chen YW, Zhang F, et al. Astragalus polysaccharides combined with cisplatin decreases the serum levels of CD44 and collagen type Ⅳ and hyaluronic acid in mice bearing Lewis lung cancer[J]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi, 2015, 31(7): 909-13. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xbyfzmyxzz201507010
[5] 吴海良.气阴两虚型肺癌动物模型的建立和评价[J].浙江中西医结合杂志, 2012, 22(12): 941-2. doi: 10.3969/j.issn.1005-4561.2012.12.009 Wu HL. Establishment and evaluation of lung cancer animal model with both Qi-and yin-deficiency in traditionan Chinese medicine[J]. Zhejiang Zhong Xi Yi Jie He Za Zhi, 2012, 22(12): 941-2. doi: 10.3969/j.issn.1005-4561.2012.12.009
[6] 陈奇.中药药理研究方法学[M]. 3版.北京:人民卫生出版社, 2011: 33-4. Chen Q. Chinese medicine pharmacology research methodology[M]. 3rd ed. Beijing: People's Medical Publishing House, 2011: 33-4.
[7] 金正均.合并用药中的相加[J].中国药理学报, 1980, 1(1): 70-1. http://www.oalib.com/references/14726431 Jin ZJ. Addition of drugs in combination[J]. Zhongguo Yao Li Xue Bao, 1980, 1(1): 70-1. http://www.oalib.com/references/14726431
[8] 周岱翰.中医肿瘤学[M].北京:中国中医药出版社, 2011: 9-15. Zhou DH, Chinese medicine oncology[M]. Beijing: Chinese Medicine Publishing House, 2011: 9-15.
[9] 唐文婷, 东方, 陈璇, 等.黄芪多糖抗肿瘤作用机制及其影响因素[J].中国民族民间医药, 2013, 22(24): 11, 15. http://d.old.wanfangdata.com.cn/Periodical/zgmzmjyyzz201324007 Tang WT, Dong F, CHeng X. et al. Antitumor mechanism of Astragalus Polysaccharides and its influencing factors[J]. Zhongguo Min Zu Min Jian Yi Yao, 2013, 22(24): 11, 15. http://d.old.wanfangdata.com.cn/Periodical/zgmzmjyyzz201324007
[10] 朱丹丹, 周大明, 赵九军.调强放射治疗联合黄芪多糖注射液治疗头颈部肿瘤的临床疗效[J].实用医学杂志, 2014, 30(10): 1652-4. doi: 10.3969/j.issn.1006-5725.2014.10.047 Zhu DD, Zhou DM, Zhao JJ. Clinical observation of IMRT therapy combined with astragalus polysaccharide injection on cervicoce-rebral tumor treatment[J]. Shi Yong Yi Xue Za Zhi, 2014, 30(10): 1652-4. doi: 10.3969/j.issn.1006-5725.2014.10.047
[11] 黄惠风, 钱建业, 谢少茹.黄芪多糖对人胃癌细胞MKN45诱导凋亡和细胞周期的影响[J].实用临床医药杂志, 2010, 14(19): 17-20. https://www.wenkuxiazai.com/doc/6ca2257b0b1c59eef8c7b4ad.html Huang HF, Qian JY, Xie SR. Effects of Astragalus Polysaccharides on apoptosis and cell cycle in human gastric carcimoma cell line MKN45[J]. Shi Yong Lin Chuang Yi Yao Za Zhi, 2010, 14(19): 17-20. https://www.wenkuxiazai.com/doc/6ca2257b0b1c59eef8c7b4ad.html
[12] Guo L, Bai SP, Zhao L, et al. Astragalus polysaccharide injectionintegrated with vinorelbine and cisplatin for patients with advanced non-small cell lung cancer: effects on quality of life and survival[J]. Med Oncol, 2012, 29(3): 1656-62. doi: 10.1007/s12032-011-0068-9
[13] Yamada T, Das Gupta TK, Beattie CW. p28-mediated Activation ofp53 in G2/M Phase of the Cell Cycle Enhances the Efficacy of DNA Damaging and Antimitotic Chemotherapy[J]. Cancer Res, 2016, 76(8): 2354-65. doi: 10.1158/0008-5472.CAN-15-2355
[14] 吕春秀, 汪治宇.金雀异黄素诱导肿瘤细胞凋亡的研究进展[J].实用癌症杂志, 2011, 26(1): 102-4. http://med.wanfangdata.com.cn/Paper/Detail/PeriodicalPaper_syazzz201101040 Lv CX, Wang ZY. Research progress of genistein induced apoptosis in tumor cells[J]. Shi Yong Ai Zheng Za Zhi, 2011, 26(1): 102-4. http://med.wanfangdata.com.cn/Paper/Detail/PeriodicalPaper_syazzz201101040
[15] 高燕, 林莉萍, 丁健.细胞周期调控的研究进展[J].生命科学, 2005, 17(4): 318-22. http://d.old.wanfangdata.com.cn/Periodical/smkx200504009 Gao Y, Lin LP, Ding J. A review: cell cycle regulation[J]. Sheng Ming Ke Xue, 2005, 17(4): 318-22. http://d.old.wanfangdata.com.cn/Periodical/smkx200504009
[16] 张树涌, 张歌, 曹勇, 等.复方桂术胶囊对小鼠Lewis肺癌的抑制及TNF-α和IFN-γ的影响[J].中成药, 2011, 33(6): 1046-9. http://d.old.wanfangdata.com.cn/Periodical/zhongcy201106039 Zhang SY, Zhang G, Cao Y, et al. Inhibition of Lewis lung cancer in mice and the effect of IFN-γ and TNF-α on lung cancer[J]. Zhong Cheng Yao, 2011, 33(6): 1046-9. http://d.old.wanfangdata.com.cn/Periodical/zhongcy201106039
[17] Cao J, Chen C, Zeng L, et al. Elevated plasma IL-22 levels correla-ted with Th1 and Th22 cells in patients with immune thrombocytopenia[J]. Clin Immunol, 2011, 14(1): 121-3. https://www.researchgate.net/publication/262884858_Circulating_Th17_Th22_and_Th1_Cells_Are_Elevated_in_the_Guillain-Barre_Syndrome_and_Downregulated_by_IVIg_Treatments
[18] Erdman SE, Rao VP, Poutahidis T, et al. Nitric oxide and TNF-alpha trigger colonic in flammation and carcinogenesis in helicobacter hepaticus-infected, Rag2-deficient mice[J]. Proc Natl Acad Sci U S A, 2009, 106(4): 1027-32. doi: 10.1073/pnas.0812347106
[19] 苏晓川, 王义生.电针联合玻璃酸钠注射对膝骨关节炎患者关节液中IL-1β、TNF-α的影响及疗效[J].实用医学杂志, 2012, 28(15): 2546-8. doi: 10.3969/j.issn.1006-5725.2012.15.028 Su XC, Wang YS. Effect of electroacupuncture combined with sodium hyaluronate injection on IL-1β-and TNF-α in joint fluid of patients with knee osteoarthritis and its curative effect[J]. Shi Yong Yi Xue Za Zhi, 2012, 28(15): 2546-8. doi: 10.3969/j.issn.1006-5725.2012.15.028
[20] Gu JW, Makey KL, Tucker KB, et al. EGCG, a majorgreen tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF-1α and NF-κB, and VEGF expression[J]. Vasc Cell, 2013, 5(1): 9. doi: 10.1186/2045-824X-5-9
[21] 邓碧凡, 廖敏, 邱荣敏, 等.缺氧对喉癌Hep-2细胞HIF-1α、GLUT-1、MMP-2表达的影响[J].肿瘤防治研究, 2016, 43(8): 663-7. http://www.zlfzyj.com/CN/abstract/abstract8809.shtml Deng BF, Liao M, Qiu RM, et al. Effect of Hypoxia on Expression of HIF-1α, GLUT-1 and MMP-2 in Laryngeal Carcinoma Cell Line Hep-2[J]., 2016, 43(8): 663-7. http://www.zlfzyj.com/CN/abstract/abstract8809.shtml
[22] Yu L, Deng L, Li J, et al. The prognostic value of vascular endothelial growth factor in ovarian cancer: a systematic review and meta-analysis[J]. Gynecol Oncol, 2013, 128(2): 391-6. doi: 10.1016/j.ygyno.2012.11.002
[23] 梁晶, 贾新凤, 韩福才.肺癌患者外周血中MMP-7 mRNA、sMICA、VEGF的表达及其与侵袭转移的关系[J].肿瘤防治研究, 2016, 43(6): 508-12. http://www.zlfzyj.com/CN/abstract/abstract8777.shtml Liang J, Jia XF, Han FC. Expressions of MMP-7 mRNA, sMICA, VEGF in Peripheral Blood of Lung Cancer Patients and Their Relationships with Invasion and Metastasis[J]. Zhong Liu Fang Zhi Yan Jiu, 2016, 43(6): 508-12. http://www.zlfzyj.com/CN/abstract/abstract8777.shtml
[24] 蒋晓东, 戴鹏, 宋大安, 等. HIF-1α、VEGF、VEG-FR2在非小细胞肺癌组织中的表达及临床意义[J].临床肺科杂志, 2011, 16(3): 386-8. http://www.wenkuxiazai.com/doc/71ed0796ec3a87c24028c4f1.html Jiang XD, Dai P, Song DA, et al. Expression of HIF-1α, VEGF and VEGFR2 in non small cell lung cancer their clincical significance[J]. Lin Chuang Fei Ke Za Zhi, 2011, 16(3): 386-8. http://www.wenkuxiazai.com/doc/71ed0796ec3a87c24028c4f1.html
[25] 杨栋, 张培彤, 王耀焓, 等.川芎嗪对PG干细胞样细胞VEGF、HIF-1α蛋白表达的影响[J].中国肿瘤, 2015, 24(3): 234-9. doi: 10.11735/j.issn.1004-0242.2015.03.A014 Yang D, Zhang PT, Wang YH, et al. Effect of TMP on the expression of VEGF and HIF-1αof PG CSC like cells[J]. Zhongguo Zhong Liu, 2015, 24(3): 234-9. doi: 10.11735/j.issn.1004-0242.2015.03.A014
[26] 左顺庆, 王建军, 郭家龙, 等.缺氧诱导因子-1α与血管内皮生长因子-C在非小细胞肺癌中的表达及相关性[J].实用医学杂志, 2009, 25(1): 59-61. doi: 10.3969/j.issn.1006-5725.2009.01.022 Zuo SQ, Wang JJ, Guo JL, et al. Expression of correlation of HIF-αand VEGF-C in non small cell lung cancer[J]. Shi Yong Yi Xue Za Zhi, 2009, 25(1): 59-61. doi: 10.3969/j.issn.1006-5725.2009.01.022
[27] 刘旭之, 宋卓. MMP-2、NM23在非小细胞肺癌组织和癌旁组织中表达及其临床意义[J].实用癌症杂志, 2010, 25(3): 255-8. http://www.cnki.com.cn/Article/CJFDTOTAL-SYAZ201003012.htm Liu XZ, Song Z. The expression and its clinical significance of MMP-2 and NM23 in the tissues from non-small cell lung cancer and the paracancerous tissues[J]. Shi Yong Ai Zheng Za Zhi, 2010, 25(3): 255-8. http://www.cnki.com.cn/Article/CJFDTOTAL-SYAZ201003012.htm



 下载:
下载: