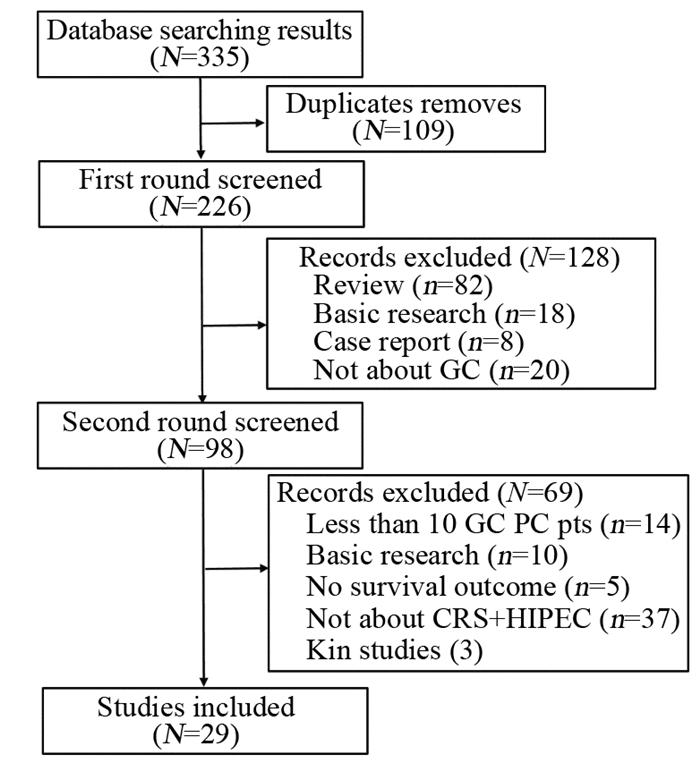
Cytoreductive Surgery plus Hyperthermic Intraperitoneal Chemotherapy on Gastric Cancer Peritoneal Carcinomatosis: A Systemic Analysis
-
摘要:目的
系统分析肿瘤细胞减灭术(cytoreductive surgery, CRS)加腹腔热灌注化疗(hyperthermic intraperitoneal chemotherapy, HIPEC)治疗胃癌腹膜癌(gastric cancer peritoneal carcinomatosis, GC PC)的应用现状及发展方向。
方法检索CRS+HIPEC治疗GC PC相关临床研究文献,提取生存及安全性相关数据,进行系统分析。
结果GC PC的自然病程不超过5月,CRS+HIPEC可延长GC PC患者总生存期(overall survival, OS)。在前瞻性临床研究中CRS+HIPEC组中位OS为11.0月,在回顾性临床研究中CRS+HIPEC组中位OS为13.3月。CRS+HIPEC治疗GC PC围手术期死亡率低于6.5%,严重不良事件发生率无显著上升。
结论CRS+HIPEC治疗GC PC疗效显著,有望成为部分经选择的GC PC患者的首选治疗。
Abstract:ObjectiveTo evaluate the clinical trials of cytoreductive surgery (CRS) plus hyperthermic intraperitoneal chemotherapy (HIPEC) on gastric cancer peritoneal carcinomatosis (GC PC).
MethodsThe published clinical trials of CRS+HIPEC on GC PC were critically evaluated, with survival and safety as the primary endpoints.
ResultsThe natural course of GC PC was < 5 months. CRS+HIPEC could improve the overall survival (OS). In prospective studies, the median OS was 11.0 months in the CRS+HIPEC group. In retrospective studies, the median OS was 13.3 months in the CRS+HIPEC group. The perioperative mortality was less than 6.5%, and there was no statistically significant increase in serious adverse events directly attributable to CRS+HIPEC.
ConclusionCRS+HIPEC is a promising integrated treatment strategy for GC PC to produce improved treatment efficacy, and should be recommended as the first treatment choice for selected patients with GC PC.
-
0 引言
胃癌是世界上癌症相关死亡的三大常见原因之一。目前根治性手术以及放化疗等辅助治疗方案的联合应用,胃癌患者的生存期已显著延长[1]。但是大多数患者最终会对化疗药物产生耐药现象,胃癌患者并不能通过传统的治疗方法受益,因此寻找新的治疗策略是当务之急[2]。有研究发现miR-451可促进消化道肿瘤的发生发展,然而,miR-451是否参与胃癌对5-Fu耐药的调节仍未可知[3]。MRP通过细胞外和细胞内膜运输各种分子,涉及多重耐药性[4]。该蛋白质作为多特异性有机阴离子转运蛋白起作用,是广泛表达于人体各组织中的耐药基因,在调节肿瘤对药物的反应性过程中发挥重要作用。在非小细胞肺癌中,MRP可作为耐药基因影响患者对铂类药物的敏感度[5]。本研究拟通过RT-PCR检测miR-451在胃癌中的表达水平,在细胞水平上分析其对胃癌细胞增殖、迁移能力的影响,并揭示其可能参与的分子调控路径。
1 资料与方法
1.1 资料
293T细胞、胃癌亲本细胞株(BGC-823、SGC-790、MKN-4、MKN-45、MKN-28)及BGC-823耐药细胞株、MKN-4耐药细胞株、MKN-28耐药细胞株均购自ATCC(美国);DMEM细胞培养基购自SIGMA-ALDRICH公司(德国);胎牛血清购自Gibco公司(美国);CCK8试剂盒购自MCE公司(中国);psPAX2、pMD2G、pLenti-miR-451 or pLenti-control均购自优宝生物(中国);miRNA-451引物、U6引物、GAPDH引物、MRP引物、NC inhibitor以及miR-451 inhibitor购自广州锐博生物科技有限公司(中国);RNA提取试剂盒购自TaKaRa公司(日本);RNA反转录试剂盒(Revert Aid First Strand cDNA Synthesis Kit)以及miRNA Universal SYBR® qPCR Master Mix试剂盒购自南京诺唯赞生物科技有限公司(中国);一抗GAPDH及辣根过氧化物酶标记的羊抗兔/鼠二抗均购于三鹰生物科技有限公司(中国);Western blot试剂盒及BCA蛋白浓度测量试剂盒购自碧云天生物技术公司(中国)。
1.2 组织来源
收集西安医学院第一附属医院胃癌患者20例,取癌组织和癌旁组织样本后立即置于-80℃液氮中冷冻待用。
1.3 细胞培养
293T细胞、胃癌细胞系(BGC-823、SGC-790、MKN-4、MKN-45和MKN-28)培养于含10%FBS的DMEM中,BGC-823耐药细胞株、MKN-4耐药细胞株、MKN-28耐药细胞株培养于含1 mg/L的10%FBS的DMEM中,且均在含饱和湿度、5%CO2、37℃的细胞培养箱中培养。
1.4 病毒包装
胰酶消化收集对数期生长的293T细胞,待细胞生长至60%时,按照病毒包装以及转染试剂要求,pLenti-control/pLenti-miR-451:psPAX2: pMD2G=4:3:1混合后,转染入293T细胞,6 h后更换新鲜DMEM培养基,48 h收集细胞上清液用于转染胃癌细胞(MKN-4/MKN-28/BGC-823耐药细胞株)。
1.5 构建稳定过表达细胞系
胰酶消化生长于对数期的胃癌细胞系(MKN-4/MKN-28/BGC-823耐药细胞株),待细胞生长至50%时,按要求转染胃癌细胞,24 h后更换新鲜培养基,持续使用嘌呤霉素(1 mg/L)筛选,2周后,荧光定量PCR检测过表达效率。
1.6 细胞转染
取对数生长期的细胞,以105个/孔密度接种于6孔板中,待细胞融合度达到80%时,采用LipofectamineTM 3000将miR-451 inhibitor转染入胃癌细胞系(MKN-4、MKN-28耐药细胞株),继续培养4 h后,更换含FBS的DMEM培养基继续培养以供后续实验。
1.7 RNA提取及荧光定量
从不同胃癌组织及胃癌细胞系(BGC-823、SGC-790、MKN-4、MKN-45、MKN-28)中提取RNA用于测定胞内miR-451的相对表达量。按照RNA提取试剂盒要求提取RNA,按照反转录试剂盒要求将其反转录为CDNA,合成的CDNA按照miRNA Universal SYBR®qPCR Master Mix试剂盒要求进行PCR扩增,以U6为内参检测miR-451的表达,以GAPDH为内参检测MRP的表达。U6正向引物序列:5’-GCTTGCTTCAGCAGCACATA-3’,反向引物:5’-AAAAACATGGAACTCTTCACG-3’;miR-45正向引物:5’-CCGAAACCGTTACCATTAC-3’,反向引物:5’-GTGCAGGGTCCGAGGT-3’;GADPH正向引物:5’-GGCATGGACTGTGGTCATGAG-3’,反向引物:5’-TGCACCACCAACTGTTAGC-3’;MRP正向引物:5’-CCCGCTCTGGGACTGGAA-3’,反向引物:5’-ACTTGTTCCGACGTGTCCTC-3’。以上实验均重复三次。
1.8 Western blot检测MRP的表达
取处理好的细胞,加入RIPA裂解液后冰浴30 min使细胞充分裂解,120 000 r/min 4℃,离心10 min,取上清液,蛋白定量,SDS-PAGE电泳2 h,NC膜转膜2.5 h,室温用含5%脱脂奶粉的TBST封闭1 h,一抗MRP以1:2 000配制,β-actin以1:5 000配制,室温孵育4 h,TBST溶液洗3次,每次10 min,二抗以1:5 000稀释配制,室温孵育1 h,TBST溶液洗3次,每次10 min,曝光显影。
1.9 荧光素酶实验
将稳定过表达miR-451的BGC-823、MKN-4耐药细胞株以及对照细胞株接种于24孔板(1.0×105个/孔)。24 h后,将pGL3-MRP-3’野生型以及突变型质粒转染稳定过表达的上述细胞,海参荧光素酶作为内参,24 h后检测荧光素酶活性差异。
1.10 CCK8检测细胞活力
取稳定过表达miR-451的BGC-823、MKN-4耐药细胞株,接种于96孔板(2×103个/孔)中,每组设4个平行复孔。分别加入0、2、4、6、8、10 g/ml 5-Fu,48 h后避光加入20 μl CCK-8溶液,常规孵育2.5 h后,多功能酶标仪检测450 nm波长处的吸光度(A)值,计算细胞增殖能力。
1.11 统计学方法
应用SPSS16.0软件进行统计分析,相关性分析符合Pearson相关性分析方法,计量数据用均数±标准差(x±s)表示,组间比较采用t检验,每组实验至少重复3次,P < 0.05为差异有统计学意义。
2 结果
2.1 miR-451在不同胃癌组织及胃癌细胞系中的表达
RT-PCR检测结果显示耐药胃癌组织中miR-451表达量明显低于非耐药胃癌组织(P=0.00043)。胃癌细胞株MKN-4 miR-451相对表达量为9.26±1.02,MKN-28细胞系中miR-451相对表达量为63.42±6.84,见图 1。
2.2 miR-451过表达抑制胃癌细胞增殖且降低胃癌细胞对5-Fu的耐药性
构建miR-451稳定过表达MKN-4耐药细胞株,结果显示miR-451显著过表达。miR-451 inhibitor转染MKN-28耐药细胞株,结果显示miR-451表达明显受到抑制,见图 2A。在不同5-Fu浓度刺激下,过表达miR-451可明显降低耐药细胞的增殖能力,抑制miR-451表达可促进耐药细胞系的增殖能力,见图 2B。
2.3 MiR-451对MRP表达的调节作用
miRBase、TargetScan、miRanda、miRDB生物数据库分析结果显示MRP为miR-451的靶基因,见图 3A。随之,构建MRP野生型以及突变型荧光报告质粒,见图 3B。荧光素酶报告实验证明,在转染野生型的双荧光报告质粒后,miR-451过表达使荧光素酶相对活性明显降低(P < 0.01),而突变型则无限制变化,见图 3C~D。以上结果证明miR-451靶向MRP的3’UTR。
![]() 图 3 miR-451的靶基因分析Figure 3 Analysis of miR-451 target geneA: Bioinformatics analysis results showed that MRP was the target gene of miR-451; B: We constructed 3'UTR wild-and mutant-type dual fluorescein reporter plasmids of MRP; C, D: miR-451 regulated the fluorescence activity of wild-type and mutant MRP reporter plasmids in BGC-823 and MKN-4 cells
图 3 miR-451的靶基因分析Figure 3 Analysis of miR-451 target geneA: Bioinformatics analysis results showed that MRP was the target gene of miR-451; B: We constructed 3'UTR wild-and mutant-type dual fluorescein reporter plasmids of MRP; C, D: miR-451 regulated the fluorescence activity of wild-type and mutant MRP reporter plasmids in BGC-823 and MKN-4 cells2.4 miR-451调控MRP的表达
miR-451过表达可明显抑制MRP转录(P=0.00075),见图 4A。20例胃癌组织中miR-451以及MRP mRNA水平显著负相关,见图 4B。Western blot检测结果显示过表达miR-451可显著降低MRP蛋白水平(P=0.000045),而miR-451 inhibitor抑制miR-451表达后,其蛋白水平出现明显上调(P=0.00029),见图 4C。
2.5 过表达MRP可促进耐药细胞系对5-Fu的耐药性
在稳定过表达miR-451的耐药细胞系BGC-283以及MKN-4中过表达MRP,Western blot实验证明了MRP的过表达效率(P=0.000062)。相对单一过表达miR-451的胃癌细胞系,miR-451可明显增加胃癌耐药细胞系BGC-283以及MKN-4对5-Fu的敏感度,其细胞增殖能力明显低于对照组;而CCK8检测结果显示,相对于单一过表达miR-451,过表达miR-451和MRP可明显增加细胞的增殖能力(P=0.00032),见图 5。说明MRP可增加胃癌细胞对5-Fu的耐受能力。
3 讨论
药物抵抗是目前胃肠道肿瘤治疗的一大难题,现有的胃肠道肿瘤治疗方案难以进一步提高患者的生存期,开发新的药物靶点是肿瘤治疗的当务之急[6]。MicroRNA作为非编码RNA参与肿瘤细胞的增殖、分化以及凋亡,有文献证明了microRNA作为治疗靶点用于肿瘤等疾病的可行性[7]。Tsuchiya等[8]发现miR-451有助于上皮细胞基底外侧极性的形成。Ribeiro-dos-Santos等[9]通过高通量测序建立了人胃组织miRNA表达谱,结果发现miR-31、miR-9b、miR-148a以及miR-451在胃癌组织中高度表达,说明miR-451可作为药物治疗的靶点用于胃癌的辅助治疗。在非小细胞肺癌中,miR-451可通过抑制AKT信号通路的激活增加肺癌对顺铂的敏感度[10]。Liu等[11]发现过表达miR-451能够降低肺癌细胞对伊马替尼的耐药作用,提高TKI的疗效。此外,在乳腺癌中,他莫昔芬也可诱导miR-451的上调,降低肿瘤细胞的抵抗效果[12]。本研究发现,miR-451参与胃癌细胞对5-Fu的耐药作用。RT-PCR检测结果显示miR-451在非耐药胃癌中表达量高于耐药胃癌组织,CCK8实验显示miR-451参与调节胃癌细胞对5-Fu的耐药作用,证明miR-451可明显降低细胞对5-Fu的耐药性,并通过生物信息学分析的方法证明了耐药基因MRP为miR-451的靶基因,荧光素酶报告实验显示上调miR-451可明显抑制野生型MRP的荧光强度,RT-PCR以及Western blot实验验证了上调miR-451可抑制MRP的表达,而抑制miR-451可促进MRP的表达,表明miR-451通过与MRP的3'UTR结合直接调节其表达。MRP作为调节肿瘤细胞对各种药物的耐药作用已被证实,O'Meara等[13]发现MRP可能参与HIV耐药,MRP可通过影响抗HIV药物转运至转染的细胞中抑制药物对HIV的杀伤。Boumendjel等[14]发现MRP也可影响抗结核杆菌药物的转运,增加结核杆菌的耐药现象。以上说明MRP在耐药方面发挥广泛的作用。
本研究阐述了miR-451异常表达介导了胃癌细胞系对5-Fu的耐药作用,并通过生物信息学相关分析,证明了miR-451通过靶基因MRP影响胃癌细胞对5-Fu的耐药作用,为目前寻找胃癌耐药方面的治疗提供了新的思路以及依据。
-
表 1 CRS+HIPEC治疗GC PC临床研究基本信息
Table 1 Major characteristics of CRS+HIPEC studies for GC PC

表 2 CRS+HIPEC治疗GC PC生存相关结果
Table 2 Survival outcomes of CRS+HIPEC on GC PC patients

表 3 CRS+HIPEC相关并发症发生率、死亡率及严重不良事件
Table 3 Mortality, morbidity and serious adverse events (SAEs) of CRS+HIPEC

-
[1] Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012 [J]. CA Cancer J Clin, 2015, 65(2): 87-108. doi: 10.3322/caac.21262
[2] Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015 [J]. CA Cancer J Clin, 2016, 66(2): 115-32. doi: 10.3322/caac.21338
[3] Yonemura Y, Canbay E, Li Y, et al. A comprehensive treatment for peritoneal metastases from gastric cancer with curative intent [J]. Eur J Surg Oncol, 2016, 42(8): 1123-31. doi: 10.1016/j.ejso.2016.03.016
[4] Thomassen I, van Gestel YR, van Ramshorst B, et al. Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, survival and risk factors [J]. Int J Cancer, 2014, 134(3): 622-8. doi: 10.1002/ijc.28373
[5] Li Y, Zhou YF, Liang H, et al. Chinese expert consensus on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal malignancies[J]. World J Gastroenterol, 2016, 22(30): 6906-16. doi: 10.3748/wjg.v22.i30.6906
[6] Roviello F, Marrelli D, de Manzoni G, et al. Prospective study of peritoneal recurrence after curative surgery for gastric cancer[J]. Br J Surg, 2003, 90(9): 1113-9. doi: 10.1002/(ISSN)1365-2168
[7] Cao L, Selby LV, Hu X, et al. Risk factors for recurrence in T1-2N0 gastric cancer in the United States and China[J]. J Surg Oncol, 2016, 113(7): 745-9. doi: 10.1002/jso.v113.7
[8] Lee JH, Lee CM, Son SY, et al. Laparoscopic versus open gastrectomy for gastric cancer: long-term oncologic results[J]. Surgery, 2014, 155(1): 154-64. doi: 10.1016/j.surg.2013.06.015
[9] Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study[J]. Cancer, 2000, 88(2): 358-63. doi: 10.1002/(ISSN)1097-0142
[10] Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase Ⅲ randomized clinical trial[J]. Ann Surg Oncol, 2011, 18(6): 1575-81. doi: 10.1245/s10434-011-1631-5
[11] Glehen O, Gilly FN, Arvieux C, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy[J]. Ann Surg Oncol, 2010, 17(9): 2370-7. doi: 10.1245/s10434-010-1039-7
[12] Rudloff U, Langan RC, Mullinax JE, et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial[J]. J Surg Oncol, 2014, 110(3): 275-84. doi: 10.1002/jso.23633
[13] Yang XJ, Li Y, Yonemura Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat gastric cancer with ascites and/or peritoneal carcinomatosis: Results from a Chinese center[J]. J Surg Oncol, 2010, 101(6): 457-64. doi: 10.1002/jso.v101:6
[14] Glehen O, Schreiber V, Cotte E, et al. Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer[J]. Arch Surg, 2004, 139(1): 20-6. doi: 10.1001/archsurg.139.1.20
[15] Beaujard AC, Glehen O, Caillot JL, et al. Intraperitoneal chemohyperthermia with mitomycin C for digestive tract cancer patients with peritoneal carcinomatosis[J]. Cancer, 2000, 88(11): 2512-9. doi: 10.1002/(ISSN)1097-0142
[16] Wu HT, Peng KW, Ji ZH, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with lobaplatin and docetaxel to treat synchronous peritoneal carcinomatosis from gastric cancer: Results from a Chinese center[J]. Eur J Surg Oncol, 2016, 42(7): 1024-34. doi: 10.1016/j.ejso.2016.04.053
[17] Tu Y, Tian Y, Fang Z, et al. Cytoreductive surgery combined with hyperthermic intraperitoneal chemoperfusion for the treatment of gastric cancer: A single-centre retrospective study[J]. Int J Hyperthermia, 2016, 32(6): 587-94. doi: 10.1080/02656736.2016.1190987
[18] Boerner T, Graichen A, Jeiter T, et al. CRS-HIPEC prolongs survival but is not curative for patients with peritoneal carcinomatosis of gastric cancer[J]. Ann Surg Oncol, 2016, 23(12): 3972-77. doi: 10.1245/s10434-016-5306-0
[19] Passot G, Vaudoyer D, Villeneuve L, et al. What made hyperthermic intraperitoneal chemotherapy an effective curative treatment for peritoneal surface malignancy: A 25-year experience with 1, 125 procedures[J]. J Surg Oncol, 2016, 113(7): 796-803. doi: 10.1002/jso.v113.7
[20] Desantis M, Bernard JL, Casanova V, et al. Morbidity, mortality, and oncological outcomes of 401 consecutive cytoreductive procedures with hyperthermic intraperitoneal chemotherapy (HIPEC)[J]. Langenbecks Arch Surg, 2015, 400(1): 37-48. doi: 10.1007/s00423-014-1253-z
[21] Yarema RR, Ohorchak MA, Zubarev GP, et al. Hyperthermic intraperitoneal chemoperfusion in combined treatment of locally advanced and disseminated gastric cancer: results of a single-centre retrospective study[J]. Int J Hyperthermia, 2014, 30(3): 159-65. doi: 10.3109/02656736.2014.893451
[22] Magge D, Zenati M, Mavanur A, et al. Aggressive locoregional surgical therapy for gastric peritoneal carcinomatosis[J]. Ann Surg Oncol, 2014, 21(5): 1448-55. doi: 10.1245/s10434-013-3327-5
[23] Müller H, Hotopp T, Tofeili A, et al. Systemic chemotherapy using FLOT-regimen combined with cytoreductive surgery plus HIPEC for treatment of peritoneal metastasized gastric cancer[J]. Hepatogastroenterology, 2014, 61(131): 703-6. http://europepmc.org/abstract/MED/26176060
[24] Konigsrainer I, Horvath P, Struller F, et al. Initial clinical experience with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in signet-ring cell gastric cancer with peritoneal metastases[J]. J Gastric Cancer, 2014, 14(2): 117-22. doi: 10.5230/jgc.2014.14.2.117
[25] Canbay E, Mizumoto A, Ichinose M, et al. Outcome data of patients with peritoneal carcinomatosis from gastric origin treated by a strategy of bidirectional chemotherapy prior to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in a single specialized center in Japan[J]. Ann Surg Oncol, 2014, 21(4): 1147-52. doi: 10.1245/s10434-013-3443-2
[26] Schildberg CW, Weidinger T, Hohenberger W, et al. Metastatic adenocarcinomas of the stomach or esophagogastric junction (UICC stage Ⅳ) are not always a palliative situation: a retrospective analysis[J]. World J Surg, 2014, 38(2): 419-25. doi: 10.1007/s00268-013-2293-1
[27] Kang LY, Mok KT, Liu SI, et al. Intraoperative hyperthermic intraperitoneal chemotherapy as adjuvant chemotherapy for advanced gastric cancer patients with serosal invasion[J]. J Chin Med Assoc, 2013, 76(8): 425-31. doi: 10.1016/j.jcma.2013.04.004
[28] Hultman B, Lind P, Glimelius B, et al. Phase Ⅱ study of patients with peritoneal carcinomatosis from gastric cancer treated with preoperative systemic chemotherapy followed by peritonectomy and intraperitoneal chemotherapy[J]. Acta Oncol, 2013, 52(4): 824-30. doi: 10.3109/0284186X.2012.702925
[29] Shen P, Stewart JH 4th, Levine EA. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: non-colorectal indications[J]. Curr Probl Cancer, 2009, 33(3): 168-93. doi: 10.1016/j.currproblcancer.2009.06.005
[30] Scaringi S, Kianmanesh R, Sabate JM, et al. Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: a single western center experience[J]. Eur J Surg Oncol, 2008, 34(11): 1246-52. doi: 10.1016/j.ejso.2007.12.003
[31] Zhu ZG, Tang R, Yan M, et al. Efficacy and safety of intraoperative peritoneal hyperthermic chemotherapy for advanced gastric cancer patients with serosal invasion. A long-term follow-up study[J]. Dig Surg, 2006, 23(1-2): 93-102. doi: 10.1159/000093778
[32] Yonemura Y, Kawamura T, Bandou E, et al. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion[J]. Br J Surg, 2005, 92(3): 370-5. doi: 10.1002/(ISSN)1365-2168
[33] Hall JJ, Loggie BW, Shen P, et al. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer[J]. J Gastrointest Surg, 2004, 8(4): 454-63. doi: 10.1016/j.gassur.2003.12.014
[34] Fujimura T, Yonemura Y, Nakagawara H, et al. Subtotal peritonectomy with chemohyperthermic peritoneal perfusion for peritonitis carcinomatosa in gastrointestinal cancer[J]. Oncol Rep, 2000, 7(4): 809-14. http://www.oalib.com/references/7734737
[35] Hirose K, Katayama K, Iida A, et al. Efficacy of continuous hyperthermic peritoneal perfusion for the prophylaxis and treatment of peritoneal metastasis of advanced gastric cancer: evaluation by multivariate regression analysis[J]. Oncology, 1999, 57(2): 106-14. doi: 10.1159/000012016
[36] Yonemura Y, Fujimura T, Nishimura G, et al. Effects of intraoperative chemohyperthermia in patients with gastric cancer with peritoneal dissemination [J]. Surgery, 1996, 119(4): 437-44. doi: 10.1016/S0039-6060(96)80145-0
[37] Yonemura Y, Fujimura T, Fushida S, et al. Hyperthermo-chemotherapy combined with cytoreductive surgery for the treatment of gastric cancer with peritoneal dissemination[J]. World J Surg, 1991, 15(4): 530-5. doi: 10.1007/BF01675656
[38] Fujimoto S, Takahashi M, Mutou T, et al. Improved mortality rate of gastric carcinoma patients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined with surgery[J]. Cancer, 1997, 79(5): 884-91. doi: 10.1002/(ISSN)1097-0142
[39] Averbach AM, Jacquet P. Strategies to decrease the incidence of intra-abdominal recurrence in resectable gastric cancer[J]. Br J Surg, 1996, 83(6): 726-33. doi: 10.1002/(ISSN)1365-2168
[40] Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: Progress toward a new standard of care[J]. Cancer Treat Rev, 2016, 48: 42-9. doi: 10.1016/j.ctrv.2016.06.007
[41] Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer[J]. Cancer Chemother Pharmacol, 1999, 43(Suppl):S15-25. doi: 10.1007/s002800051093
[42] Sugarbaker PH. Cytoreductive surgery and peri-operative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome[J]. Eur J Surg Oncol, 2001, 27(3): 239-43. doi: 10.1053/ejso.2000.1038
[43] Lemoine L, Sugarbaker P, Van der Speeten K. Drugs, doses, and durations of intraperitoneal chemotherapy: standardising HIPEC and EPIC for colorectal, appendiceal, gastric, ovarian peritoneal surface malignancies and peritoneal mesothelioma[J]. Int J Hyperthermia, 2017, 33(5): 582-92. doi: 10.1080/02656736.2017.1291999
-
期刊类型引用(1)
1. 冯晓慧,朱正秋. NLR对晚期食管鳞癌一线免疫治疗疗效的预测价值. 医学研究杂志. 2023(07): 157-160+123 .  百度学术
百度学术
其他类型引用(2)




 下载:
下载: