Preoperative Platelet-to-lymphocyte Ratio and Neutrophil-to-lymphocyte Ratio for Predicting Prognosis of Esophageal Cancer Patients
-
摘要:目的
探讨术前外周血血小板与淋巴细胞比值(PLR)和中性粒细胞与淋巴细胞比值(NLR)对食管癌患者预后的预测价值。
方法回顾性分析137例行食管癌根治切除术治疗的患者的临床资料。按术前PLR<120及≥120、NLR<2.0及≥2.0分组,采用Kaplan-Meier曲线法进行生存分析,比较5年无病生存率和总生存率。采用Cox多因素分析确定影响预后的独立因素。
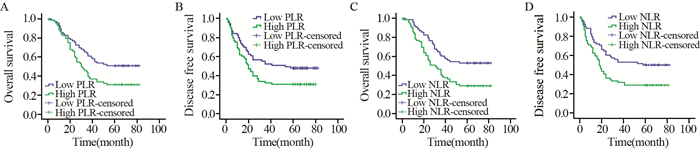
结果高PLR组的5年总生存率30.9%和无病生存率30.9%分别低于低PLR组的50.7%和47.8%(P=0.017, P=0.033)。同样,高NLR组的5年总生存率29.0%和无病生存率29.0%显著低于低NLR组的52.9%和50.0%(P=0.003, P=0.006)。多因素分析显示,分化程度、临床分期和NLR是影响食管癌患者预后的独立因素(均P<0.05)。
结论PLR和NLR可以预测食管癌患者的预后,NLR还可作为影响患者预后的独立因素。
-
关键词:
- 食管癌 /
- 血小板与淋巴细胞比值 /
- 中性粒细胞与淋巴细胞比值 /
- 无病生存率 /
- 总生存率
Abstract:ObjectiveTo investigate the prognostic value of the preoperative platelet-to-lymphocyte ratio (PLR) and the neutrophil-to-lymphocyte ratio (NLR) collected from peripheral blood of esophageal cancer (EC) patients.
MethodsThe clinical data of 137 patients who underwent esophagectomy from January 2010 to December 2011 were analyzed retrospectively. According to preoperative PLR and NLR, the patients were divided into low PLR group (PLR < 120) and high PLR group (PLR≥120) or low NLR group (PLR < 120) and high PLR group (PLR≥120). Disease free survival (DFS) and overall survival (OS) were assessed using Kaplan-Meier method. The prognostic significance of both markers was then determined by both univariate and multivariate analytical methods.
ResultsThe high PLR group had a much lower 5-year OS compared with the low PLR group (30.9% vs. 50.7%, P=0.017) as well as DFS (30.9% vs. 47.8%, P=0.033). The high NLR group had a much lower 5-year OS compared with the low NLR group (29.0% vs. 52.9%, P=0003) as well as DFS (29.0% vs. 50.0%, P=0.006). Multivariate analysis revealed that the differentiation, clinical stage and NLR were identified as independent risk factors for poor prognosis of EC patients.
ConclusionPLR and NLR might predict the prognosis of EC patients. NLR could be an independent predictive factor for EC.
-
0 引言
目前,乳腺癌严重威胁着女性的健康。在乳腺癌的转移机制中,上皮间质转化(epithelial-mesenchymal transition, EMT)是其中一个核心的生物学进程,与乳腺癌的侵袭和转移密切相关。JMJD3又称赖氨酸去甲基化酶6B(lysine demethylase 6B, KDM6B)作为一种Lys27位点三甲基化组蛋白H3(histone H3 methylated Lys27,H3K27me3)去甲基化酶[1],其异常表达与多种肿瘤的发生、发展密切相关[2-3]。JMJD3在肿瘤中是促癌基因还是抑癌基因研究报道尚不一致,有关JMJD3在乳腺癌中的作用,以及JMJD3与上皮间质转化的相关性尚未见类似报道。本研究收集120例乳腺浸润性导管癌组织标本,采用免疫组织化学法检测JMJD3和上皮间质转化相关蛋白E-cadherin、Vimentin和N-cadherin的表达,并分析其相关性及临床病理意义,以拓展和丰富人们对乳腺癌发病机制的认识。
1 资料与方法
1.1 病例资料
收集郑州人民医院2014年1月—2015年12月间收治的120例乳腺原发浸润性导管癌及相应的癌旁病理活检标本和相关临床资料。患者术前均未接受放疗或化疗。按照2012年WHO乳腺癌组织病理分级分类法对其进行分组。所有标本均经10%的甲醛固定,石蜡包埋,并经郑州人民医院病理科确诊。
1.2 主要试剂
JMJD3一抗兔抗人多克隆抗体购自英国Abcam公司,E-cadherin、Vimentin和N-cadherin一抗兔抗人单克隆抗体(即用型)、二抗及二氨基联苯胺(DAB)均为福州迈新公司产品。
1.3 免疫组织化学染色
主要步骤如下:(1)石蜡组织切片、烤片、脱蜡并水化;(2)EDTA抗原修复;(3)兔抗人单克隆抗体一抗37℃孵育2 h;(4)二抗37℃孵育20~30 min;(5)DAB显色5~10 min;(6)脱水、透明和封片。用已知JMJD3、E-cadherin、Vimentin和N-cadherin阳性的乳腺癌组织和PBS分别代替一抗作为阳性和空白对照。
1.4 免疫组织化学结果判断
JMJD3阳性信号呈棕褐色,表达于细胞核;E-cadherin和N-cadherin阳性信号为棕黄色,表达于细胞膜;Vimentin阳性信号为棕黄色,表达于细胞质。按样本中JMJD3、E-cadherin、Vimentin及N-cadherin阳性细胞占总细胞的比例分为4个等级[4]: < 5%为阴性、5%~ < 25%为弱阳性、25%~50%为中等阳性、≥50%为强阳性。其中阴性和弱阳性判断为低表达,中等阳性和强阳性判断为高表达,阳性表达率=(中等阳性+强阳性样本量)/总样本量,免疫组织化学的判读由两位高年资病理医师采用双盲法进行。
1.5 统计学方法
采用SPSS17.0统计软件进行数据处理与统计分析,JMJD3、E-cadherin、Vimentin和N-cadherin阳性表达率和病理因素的相关性采用χ2检验,蛋白间的相关性采用Spearman相关分析,以P < 0.05为差异有统计学意义。
2 结果
2.1 乳腺浸润性导管癌中JMJD3、E-cadherin、Vimentin和N-cadherin的表达
JMJD3主要定位于细胞核,在乳腺浸润性导管癌及癌旁组织中呈棕褐色,在导管癌中的阳性表达率明显低于癌旁组织,差异有统计学意义(χ2=7.326, P=0.007),见图 1、表 1。JMJD3表达较高者,组织学分化程度高,细胞黏附性强,极性较好,JMJD3表达较低者,组织学分化程度差,细胞黏附性下降,失去极性,细胞梭形更为明显,见图 2。
表 1 乳腺癌和癌旁组织中JMJD3、E-cadherin、Vimentin及N-cadherin的阳性表达(n(%))Table 1 Expression of JMJD3, E-cadherin, Vimentin and N-cadherin in breast cancer tissues and adjacent tissues (n(%))
E-cadherin在乳腺浸润性导管癌及癌旁组织中主要定位于细胞膜,为粗细相对均匀的棕褐色颗粒,乳腺浸润性导管癌中E-cadherin阳性表达率明显低于癌旁组织,差异有统计学意义(χ2=10.256, P=0.001)。Vimentin主要表达于上皮周围的间质细胞中,在癌及癌旁组织中主要定位于细胞质,为粗细不等的棕褐色颗粒,乳腺浸润性导管癌中Vimentin阳性表达率明显高于癌旁组织,差异有统计学意义(χ2=24.033, P=0.000)。N-cadherin在癌及癌旁组织中主要定位于细胞膜,为粗细相对均匀的棕褐色颗粒,乳腺浸润性导管癌中N-cadherin阳性表达率为明显高于癌旁组织,差异有统计学意义(χ2=35.281, P=0.000),见图 1、表 1。
2.2 JMJD3、E-cadherin、Vimentin和N-cadherin与乳腺浸润性导管癌临床病理特征的关系
在乳腺浸润性导管癌中,JMJD3与肿瘤直径(χ2=9.697, P=0.008)、组织学分级(χ2=27.313, P=0.000)、淋巴结转移(χ2=12.587, P=0.000)TNM分期有关(χ2=12.385, P=0.000),与乳腺癌患者年龄和肿瘤位置均无关(均P > 0.05)。E-cadherin在高分化、中分化和低分化乳腺癌中表达率依次降低,差异具有统计学意义(χ2=14.152, P=0.001);其在淋巴结有转移组中低于无淋巴结转移组,差异有统计学意义(χ2=9.903, P=0.002);其在TNM Ⅲ期、Ⅳ期组别低于Ⅰ期、Ⅱ期组别,差异具有统计学意义(χ2=20.771, P=0.000)。E-cadherin与乳腺癌患者年龄、肿瘤位置和肿瘤直径均无关(均P > 0.05)。Vimentin在高分化、中分化和低分化乳腺癌中表达率依次升高,差异具有统计学意义(χ2=28.410, P=0.000);其在淋巴结有转移组中高于无淋巴结转移组(χ2=14.090, P=0.000),TNMⅢ期、Ⅳ期组高于Ⅰ期、Ⅱ期组(χ2=13.218, P=0.000),与年龄、肿瘤位置和肿瘤直径均无关(均P > 0.05)。N-cadherin在高分化、中分化和低分化中表达率依次升高(χ2=28.410, P=0.000);其在淋巴结有转移组中高于无淋巴结转移组(χ2=17.678, P=0.000),在TNM Ⅲ期、Ⅳ期组高于Ⅰ期、Ⅱ期组(χ2=30.035, P=0.000)。N-cadherin与乳腺癌患者年龄、肿瘤位置及肿瘤直径均无关(均P > 0.05),见表 2。
表 2 乳腺癌中JMJD3、E-cadherin、Vimentin及N-cadherin的表达与临床病理特征的关系(n(%))Table 2 Correlation of JMJD3, E-cadherin, Vimentin and N-cadherin expression with clinicopathological characteristics of breast cancer patients (n(%))
2.3 乳腺浸润性导管癌中JMJD3与E-cadherin、Vimentin和N-cadherin相关性分析
采用Spearman相关分析,结果显示JMJD3和E-cadherin表达呈正相关(r=0.204, P=0.025)。JMJD3和Vimentin表达呈负相关(r=-0.467, P=0.000)。JMJD3和N-cadherin表达呈负相关(r=-0.500, P=0.000),见表 3。
表 3 乳腺癌中JMJD3与E-cadherin, Vimentin和N-cadherin的Spearman相关性分析Table 3 Spearman correlation analysis of JMJD3 expression with E-cadherin, Vimentin and N-cadherin expression in breast cancer tissues
3 讨论
JMJD3编码基因位于人类第17号染色体上,其异常表达与肿瘤的发生发展有密切关系。Tokunaga等[5]对151例结肠癌患者的术后生存分析结果表明,JMJD3低表达者术后生存期普遍较短,且回归分析表明JMJD3的低表达是患者不良预后的独立危险因素之一。国内有研究发现JMJD3过表达对胃癌细胞的增殖具有抑制作用,对细胞凋亡具有促进作用[6]。胡贺芳等[7]研究发现JMJD3表达降低可能促进肺癌的发生发展。李彦伟等[8]发现胶质瘤细胞中JMJD3较瘤旁组织表达降低,其过表达可增强胶质瘤细胞迁移能力,该机制通过影响SNAIL基因调控区H3K27me3水平而调节胶质瘤侵袭过程[9]。
JMJD3是促癌基因还是抑癌基因研究报道尚不一致,本研究采用免疫组织化学方法发现JMJD3在乳腺浸润性导管癌组织表达率较癌旁组织低,其在组织学分化差、有淋巴结转移及较高TNM分期的组织中表达率减低,提示JMJD3在乳腺癌的浸润转移中扮演了抑癌基因的角色。此外本研究结果还显示JMJD3低表达与肿瘤直径相关,肿瘤直径的大小反映细胞增殖能力,我们推测JMJD3对乳腺癌细胞增殖具有抑制作用,这与以往其在胃癌研究报道观点一致[6]。
在乳腺癌的转移机制中,EMT是其中一个核心的生物学进程。EMT的过程中,上皮细胞标志性蛋白E-cadherin减少,而间质细胞标志性蛋白Vimentin和N-cadherin表达量上升[10-12]。E-cadherin主要分布于胚胎组织和成熟组织的上皮细胞中,除具有调节胚胎组织的发育、组织形成、参与细胞与细胞间信息传递交流等作用外,对促进细胞的黏附聚集、维持上皮形态结构完整性、极性和参与分化调节也至关重要。Vimentin主要表达于间叶组织和淋巴造血细胞,在上皮中几乎不表达,当细胞出现上皮间质转化时可有表达。N-cadherin表达于神经外胚层和中胚层来源的组织,正常上皮组织不表达或低表达。N-cadherin在上皮组织中的异位表达可以影响上皮细胞的形态和行为,诱导其向间质转化,即产生EMT。
本研究结果显示从乳腺癌旁组织到乳腺癌组织JMJD3和E-cadherin阳性率逐渐减少,而Vimentin和N-cadherin阳性率逐渐增高;同时它们均与乳腺癌分化程度、TNM分期和淋巴结转移相关,表明JMJD3和EMT相关蛋白共同参与乳腺癌的发生、发展,其可能机制是通过降低肿瘤细胞间的黏附力,使乳腺癌更容易发生浸润和转移。本研究中E-cadherin、Vimentin和N-cadherin的表达情况与以往文献报道的结果基本一致[13-14]。
通过查阅国内外文献,我们发现JMJD3表达对上皮间质转化的影响在胶质瘤细胞[15]、肝癌细胞[16-17]、肾癌细胞[18]和弥漫大B细胞淋巴瘤[19]已有研究报道,其在乳腺癌中尚未见研究报道。上述研究均表明JMJD3过表达能够促进上皮间质转化过程进而促进肿瘤的浸润转移。本研究相关分析结果发现JMJD3与E-cadherin表达呈正相关,与Vimentin和N-cadherin表达呈负相关,表明JMJD3低表达可能通过下调E-cadherin及上调Vimentin和N-cadherin的表达使乳腺癌上皮细胞获得间质细胞的特性,进而促进EMT的发生。提示JMJD3阳性率较低的乳腺癌患者更易发生淋巴结转移和较差的组织学分化,而组织学分化较差的乳腺癌细胞在形态学上细胞极性消失,细胞梭形明显,黏附性下降,从形态学上表明JMJD3可能通过上皮间质转化途径参与乳腺癌的发生发展。本研究从蛋白水平对乳腺癌中JMJD3、E-cadherin和Vimentin表达进行分析,尚缺少分子生物学调节实验,还需进一步深入研究。
综上所述,乳腺浸润性导管癌中组蛋白去甲基化酶JMJD3与E-cadherin呈正相关,与Vimentin和N-cadherin呈负相关,JMJD3可能通过调节乳腺癌细胞上皮间质转化,调控乳腺癌侵袭转移,其具体机制有待后续深入研究。
-
表 1 食管癌患者不同PLR和NLR组的临床病理特征比较(n(%))
Table 1 Comparison of clinicopathological factors between esophageal cancer patients with different PLR and NLR (n(%))

表 2 食管癌患者术前外周血血常规参数和PLR与NLR的关系
Table 2 Comparison of preoperative peripheral blood parameters between esophageal cancer patients with different PLR and NLR

表 3 影响食管癌患者生存预后的单因素分析
Table 3 Univariate analysis of clinical factors for prognosis of esophageal cancer patients

表 4 影响食管癌患者生存预后的多因素Cox回归分析
Table 4 Multivariate Cox regression analysis of clinical parameters for prognosis of esophageal cancer patients

-
[1] Wang Y, Wang L, Yang Q, et al. Factors on prognosis in patients of stage T3N0M0 thoracic esophageal squamous cell carcinoma after two-field esophagectomy[J]. J Caner Res Ther, 2015, 11(Suppl1): C16-23. http://www.ncbi.nlm.nih.gov/pubmed/26323918
[2] Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015[J]. CA Cancer J Clin, 2015, 65(1): 5-29. doi: 10.3322/caac.21254
[3] Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2): 115-32. doi: 10.3322/caac.21338
[4] Liu H, Yang J, Yuan Y, et al. Regulation of Mcl-1 by constitutive activation of NF-kappa B contributes to cell viability in human esophageal squamous cell carcinoma cells[J]. BMC Cancer, 2014, 14: 98. doi: 10.1186/1471-2407-14-98
[5] Sarraf KM, Belcher E, Raevsky E, et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer[J]. J Thoac Cardiovas Surg, 2009, 137(2): 425-8. doi: 10.1016/j.jtcvs.2008.05.046
[6] Li MX, Liu XM, Zhang XF, et al. Prognostic role of neutrophil-to-lymphocyte ratio in eolorectal cancer a systematic review and meta-analysis[J]. Int J Canccr, 2014, 134(10): 2403-13. doi: 10.1002/ijc.v134.10
[7] Mano Y, Shirabe K, Yamashila Y, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepateetomy for hepatocellular carcinoma: a retrospective analysis[J]. Ann Surg, 2013, 258(2): 301-5. doi: 10.1097/SLA.0b013e318297ad6b
[8] Arab B, Bhatt VR, Phookan J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short-and long-term mortality in breast cancer patients[J]. Ann Surg Oncol, 2011, 19(1): 217-24. http://www.sciencedirect.com/science/article/pii/S0953620511603063
[9] Pichler M, Hutterer GC, Stoeckigt C, et al. Validation of the pretreatment neutrophil-lymphocyte ratio as a prognostic factor in a large European eohort of renal cell carcinoma patients[J]. Br J Cancer, 2013, 108(4): 901-7. doi: 10.1038/bjc.2013.28
[10] Shimada H, Takiguchi N, Kainuma O, et al. High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer[J]. Gastric Cancer, 2010, 13(3): 170-6. doi: 10.1007/s10120-010-0554-3
[11] Unal D, Eroglu C, Kurtul N, et al. Are Neutrophil/Lymphocyte and Platelet/Lymphocyte Rates in Patients with Non-Small Cell Lung Cancer Associated with Treatment Response and Prognosis?[J]. Asian Pac J Cancer Prev, 2013, 14(9): 5237-42. doi: 10.7314/APJCP.2013.14.9.5237
[12] 山长平, 夏重升, 杨娅, 等.术前外周血血小板与淋巴细胞比值对非小细胞肺癌患者预后的影响[J].中国肿瘤临床, 2014, 41(21): 1374-8. doi: 10.3969/j.issn.1000-8179.20141444 Shan CP, Xia CS, Yang Y, et al. Effects of preoperative blood platelet-to-lymphocyte ratio on prognosis of non-small cell lung cancer patients after surgical resection[J]. Zhongguo Zhong Liu Lin Chuang, 2014, 41(21): 1374-8. doi: 10.3969/j.issn.1000-8179.20141444
[13] Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow[J]. Lancet, 2001, 357(9255): 539-45. doi: 10.1016/S0140-6736(00)04046-0
[14] Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer[J]. Cell, 2010, 140(6): 883-99. doi: 10.1016/j.cell.2010.01.025
[15] 常立甲, 宋淑霞. stat3信号促进肿瘤免疫抑制微环境形成的研究进展[J].中国免疫学杂志, 2012, 28(2): 177-81. http://www.docin.com/p-706804290.html Chang LJ, Song SX. Stat3 signal promotes the development of tumor immunosuppressive microenvironment formation[J]. Zhongguo Mian Yi Xue Za Zhi, 2012, 28(2): 177-81. http://www.docin.com/p-706804290.html
[16] Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma[J]. World J Surg Oncol, 2014, 12: 58. doi: 10.1186/1477-7819-12-58
[17] Sun Z, Yang P. Role of imbalance between neutrophil elastase and α1-antitrypsin in cancer development and progression[J]. Lancet Oncol, 2004, 5(3): 182-90. doi: 10.1016/S1470-2045(04)01414-7
[18] Bremnes RM, Al-Shibli K, Donnem T, et al. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression and prognosis:emphasis on non-small cell lung cancer[J]. J Thorac Oncol, 2011, 6(4): 824-33. doi: 10.1097/JTO.0b013e3182037b76
[19] Sharaiha RZ, Halazun KJ, Mirza F, et al. Elevated Preoperative neutrophil: lymphocyte ratio a predictor of postoperative disease recurfence in esophageal cancer[J]. Ann Surg Oncol, 2011, 18(12): 3362-9. doi: 10.1245/s10434-011-1754-8
[20] Smith RA, Bosonnet L, Raraty M, et al. Preoperative platelet lymphocyte ratio is all Independent significant prognostic marker in resected pancreatic duetal adenocarcinoma[J]. Ann J Surg, 2009, 197(4): 466-72. doi: 10.1016/j.amjsurg.2007.12.057
[21] Smith RA, Ghaneh P, Sutton R, et al. Prognosis of resected ampullary adenocareinoma by preoperative serum CAl9-9 levels and platelet-lymphocyte ratio[J]. J Gastmintest Surg, 2008, 12(8): 1422-8. doi: 10.1007/s11605-008-0554-3
[22] Kwon HC, Kim SH, Oh SY, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer[J]. Biomarkers, 2012, 17(3): 216-22. doi: 10.3109/1354750X.2012.656705
[23] Asher V, Lee J, Innamaa A, et al. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer[J]. Clin Transl Oncol, 2011, 13(7): 499-503. doi: 10.1007/s12094-011-0687-9
[24] 金龙, 付神波, 于娇.治疗前PLR和NLR对鼻咽癌患者预后的影响[J].肿瘤防治研究, 2017, 44(7): 476-80. http://www.zlfzyj.com/CN/abstract/abstract9005.shtml Jin L, Fu SB, Yu J. Effect of NLR and PLR from Pre-treatment on Prognosis of Nasopharyngeal Carcinoma Patients[J]. Zhong Liu Fang Zhi Yan Jiu, 2017, 44(7): 476-80. http://www.zlfzyj.com/CN/abstract/abstract9005.shtml
[25] Dashevsky O, Varon D, Brill A. Platelet-derived microparticles promote invasiveness of prostate cancer cell via up regulation of MMP-2 production[J]. Int J Cancer, 2009, 124(8): 1773-7. doi: 10.1002/ijc.v124:8
[26] Jodephs, Kathryn E, Jessicav, et al. Platelets and fibrin increase metastatic potential by impending natural killer cell mediated elimination of tumor cell[J]. Blood, 2005, 105(1): 178. doi: 10.1182/blood-2004-06-2272
[27] Xie X, Luo KJ, Hu Y, et al. Prognostic value of preoperative platelet-lymphocyte and neutrophil-lymphocyte ratio in patients undergoing surgery for esophageal squamous cell cancer[J]. Dis Esophagus, 2016, 29(1): 79-85. doi: 10.1111/dote.2016.29.issue-1
[28] Jung J, Park SY, Park SJ, et al. Prognostic value of the neutrophil-to-lymphocyte ratio for overall and disease free survival in patients with surgically treated esophageal squamous cell carcinoma[J]. Tumour Biol, 2016, 37(6): 7149-54. doi: 10.1007/s13277-015-4596-3
-
期刊类型引用(2)
1. 苗晓红,袁志英,李媛,韩秀青,李光. 宫颈癌组织含Jumonji结构域的蛋白2B、造血相关PBX相互作用蛋白的表达与预后关系的分析. 中国性科学. 2023(01): 60-65 .  百度学术
百度学术
2. 徐小艳,王建君,闫琛,李川,刘微,杨金花. ALKBH5促进乳腺浸润性导管癌细胞上皮间质转化和血管生成并增强其侵袭和转移. 细胞与分子免疫学杂志. 2021(04): 355-361 .  百度学术
百度学术
其他类型引用(0)




 下载:
下载: