Expression of PTEN and EMMPRIN in Lung Adenocarcinoma Tissues and Their Correlation with EGFR Mutation
-
摘要:目的
观察肺腺癌中PTEN和EMMPRIN蛋白水平的表达,分析其与肺腺癌临床病理特征及EGFR基因突变的关系。
方法选取10例正常肺组织作为对照组,分别用免疫组织化学法和PCR法检测90例肺腺癌组织中PTEN和EMMPRIN蛋白的表达和EGFR基因突变情况。
结果肺腺癌组织中PTEN蛋白的表达显著低于正常肺组织,EMMPRIN蛋白的表达显著高于正常肺组织。PTEN和EMMPRIN表达率为40%(36/90)和80%(72/90),EGFR基因突变率为47.78%(43/90)。PTEN的表达与肺腺癌组织学类型有关(P=0.000),EMMPRIN的表达与淋巴结转移状况及TNM分期有关(均P < 0.05)。Spearman相关分析显示PTEN和EMMPRIN呈显著负相关(rp=-0.215, P=0.041), PTEN与EGFR基因突变呈正相关(rp=0.490, P=0.000),EMMPRIN与EGFR基因突变无明显相关性(rp=0.170, P=0.110)。
结论肺腺癌中PTEN和EMMPRIN蛋白可能存在某些机制共同作用促进了肺腺癌的发生发展,PTEN蛋白的表达与EGFR基因突变呈正相关。
-
关键词:
- 肺腺癌 /
- PTEN /
- EMMPRIN/CD147 /
- EGFR基因突变
Abstract:ObjectiveTo investigate the expression of PTEN and EMMPRIN and their relationships with clinicopathological factors and EGFR mutation in lung adenocarcinoma tissues.
MethodsThe expression of PTEN and EMMPRIN proteins were examined in 90 cases of lung adenocarcinoma tissues by immunohistochemical method, and 10 cases of normal lung tissues were taken as control. The mutation of EGFR was detected using PCR.
ResultsPTEN expression in lung adenocarcinoma tissues was significantly lower than that in non-neoplastic internal controls tissues. EMMPRIN expression in lung adenocarcinoma tissues was significantly higher than that in internal controls tissues. The positive rates of PTEN and EMMPRIN expression were 40%(36/90) and 80%(72/90), respectively. EGFR gene mutation rate was 47.78%(43/90). The expression of PTEN was positively correlated with the histological subtypes (P=0.000). EMMPRIN expression was positively correlated with lymph node metastasis status and TNM stage (both P=0.000). Spearman correlation analysis showed that PTEN expression was negatively correlated with EMMPRIN expression (rp=-0.215, P=0.041). PTEN expression had a positive correlation with EGFR mutation (rp=0.490, P=0.000). EMMPRIN expression had no correlation with EGFR mutation (rp=0.170, P=0.110).
ConclusionPTEN and EMMPRIN may have a synergistic effect on the progression and development of lung adenocarcinoma. PTEN expression has a positive correlation with EGFR mutation which may provide evidences for targeted therapy.
-
Key words:
- Lung adenocarcinoma /
- PTEN /
- EMMPRIN/CD147 /
- EGFR mutation
-
0 引言
宫颈癌是威胁全球女性生命健康的第二大恶性肿瘤,发病率仅次于乳腺癌,全世界每年约有50万的新发宫颈癌病例,其中80%来自发展中国家,且发病率和病死率一直居高不下,宫颈癌的治疗一直是国内外研究的重点领域[1-2]。miRNA是一类单链内源性非编码小分子RNA,能够通过与靶mRNA特异性的碱基配对引起靶mRNA的降解或者抑制其翻译,从而对基因进行转录后的表达调控。目前已经证实miRNA在生物发育和细胞分化中发挥重要作用,越来越多的研究表明,miRNA参与多种细胞生物过程与癌症的发生、发展和转移密切相关[3-5]。
自噬是真核细胞的一种非细胞凋亡性程序性死亡,又被称为Ⅱ型程序性细胞死亡。当细胞受到不良刺激如营养缺乏、内质网应激、氧化应激、细胞器损伤以及细胞死亡的情况下都可以激活自噬信号通路,在多种人类肿瘤中存在细胞自噬活性改变,自噬活性降低促进肿瘤的发生和进展[6-8]。自噬在神经退行性疾病、感染性疾病及肿瘤发生发展过程中的作用逐渐被揭示,细胞自噬防止坏死引起的无菌性炎性反应和巨噬细胞浸润,不仅可以限制肿瘤细胞生长,同时也可以抑制肿瘤侵袭转移[9-11],GFP-LC3目前被认为是自噬体特异性标签,是一种检测自噬发生常用的实验技术手段。
为了深入了解宫颈癌发生发展的分子机制和寻找新的治疗靶点,我们通过对miR-216b在宫颈癌细胞中作用的研究,为寻求宫颈癌特异性靶向诊断和治疗提供新的思路。
1 材料与方法
1.1 材料
人宫颈癌细胞系HeLa购自中国科学院上海细胞库;RPMI 1640细胞培养液及胎牛血清购自美国Life Technologies公司;GFP-LC3质粒由南京大学医学院惠赠。雷帕霉素、Beclin-1、LC-3B、GAPDH等抗体购自美国Santa Cruz公司;胰蛋白酶与胎牛血清购自美国Gibco公司;miR-216b引物、mimic和inhibitor及内参U6均购自广州锐博生物科技有限公司,转染所用阳离子脂质体LipofectamineTM 3000及Opti-MEM购自美国Invitrogen公司。
1.2 方法
1.2.1 细胞自噬率检测
将HeLa细胞接种到24孔板内,培养过夜,使细胞密度达到70%~80%。通过LipofectamineTM 3000转染GFP-LC3质粒体,6 h后换完全培养液继续培养24 h,之后将miR-216b mimic或inhibitor分别转染48 h,阳性对照组给予5 μmol/L雷帕霉素处理24 h。将处理后的细胞用PBS清洗3次,用4%多聚甲醛室温固定15 min,PBS清洗3次,荧光显微镜下观察,统计GFP-LC3的阳性自噬点数,统计对照组和处理组单个细胞中GFP-LC3荧光点数,每组至少统计60个细胞。
1.2.2 Western blot法检测自噬相关蛋白表达
将HeLa细胞均匀接种于60 mm培养皿中,药物处理后用预冷的PBS洗3遍,加入含有100 mmol/L PMSF的裂解液(RIPA)裂解细胞,冰上放置30 min充分裂解,4℃条件下12 000 r/min离心15 min,取上清液后经BCA法测定蛋白浓度。依次进行质量分数10%的SDS-PAGE凝胶电泳,使用PVDF转膜,5%脱脂奶粉封闭2 h后,4℃封闭一抗过夜,TBST清洗3次(每次5 min)后加入相应二抗,室温下孵育60 min,TBST洗3次后进行显影,实验重复三次,采用计算机软件ImageJ分析灰度值并记录。
1.2.3 miR-216b表达水平的检测
6孔板内接种HeLa细胞各6×105个。待细胞完全贴壁后用miR-216b inhibitor转染48 h,TRIzol试剂裂解细胞,按照试剂说明书提取基因组总RNA,取<0.5 μg RNA为模板进行反转录合成cDNA。按照说明书混合PCR反应体系(SYBRGreen),每管分别加入1 μl cDNA产物,仪器设置条件为:95℃ 10 min;95℃ 15 s,60℃ 1 min,共计40个循环。
1.2.4 报告基因质粒的构建与检测
构建pmirGLO荧光素酶质粒报告系统(美国Promega公司)分析miRNA与预测靶序列的结合,该质粒含有两个不同荧光素酶基因,一个为插入Beclin-1 3’UTR序列的荧光素酶基因表达载体(野生型),另一个基因为靶结合位点突变的载体质粒(突变型)。将两种质粒分别与miR-216b mimic及其阴性对照共转染HeLa细胞,每组设5个复孔,培养48 h后进行荧光素酶相对活性检测,双荧光素酶报告实验(Dual Luciferase report assay)(美国Promega公司)上机检测。
1.3 统计学方法
采用SPSS19.0统计学软件进行统计分析,经K-S检验,本研究各检测指标的数据资料呈正态分布,以均数±标准差表示,组间均数经Levene检验方差齐。采用均衡分组单因素干预多水平实验设计,处理因素作用总体差异比较均采用单因素方差分析,两两比较分别采用LSD-t检验和SNK-q检验。P<0.05被认为差异具有统计学意义。
2 结果
2.1 miR-216b抑制宫颈癌细胞自噬水平
HeLa细胞转染GFP-LC3后,再转染miR-216b mimic,以雷帕霉素作为阳性对照,在荧光显微镜下观察并对带有GFP-LC3的自噬点阳性细胞数和总细胞数进行统计。相对于对照组和雷帕霉素组的细胞,miR-216b能够明显降低细胞自噬体的数量,见图 1。Western blot检测结果显示,自噬标记蛋白Beclin1表达量减少,作为自噬发生指标的LC3-Ⅱ/LC3-I比值也同样表现出下降;miR-216b能够降低HeLa细胞自噬蛋白的表达水平,见图 2。综上结果表明,miR-216b能够抑制HeLa细胞的自噬水平。
![]() 图 1 荧光显微镜观察经miR-216b转染或雷帕霉素处理后HeLa细胞中GFP-LC3自噬点的分布变化Figure 1 Distribution of GFP-LC3 autophagy points in HeLa cells after transfection of miR-216b or rapamycin treatment observed by fluorescence microscopyA: the autophagy positive cells in normal control group,miR-216b mimic group and rapamycin group were observed by fluorescence microscope. Bright green fluorescent spots represented autophagy positive cells and the pictures below were for low power lens to corresponding groups;B: Autophagy positive cell rates in normal control group,miR-216b mimic group and rapamycin group (*: P<0.05)
图 1 荧光显微镜观察经miR-216b转染或雷帕霉素处理后HeLa细胞中GFP-LC3自噬点的分布变化Figure 1 Distribution of GFP-LC3 autophagy points in HeLa cells after transfection of miR-216b or rapamycin treatment observed by fluorescence microscopyA: the autophagy positive cells in normal control group,miR-216b mimic group and rapamycin group were observed by fluorescence microscope. Bright green fluorescent spots represented autophagy positive cells and the pictures below were for low power lens to corresponding groups;B: Autophagy positive cell rates in normal control group,miR-216b mimic group and rapamycin group (*: P<0.05)![]() 图 2 Western blot检测miR-216b抑制细胞自噬水平Figure 2 Cell autophagy inhibited by miR-216b detected by Western blotA: the expression of Beclin-1,LC3-Ⅰ and LC3-Ⅱ in normal control group,miR-216b mimic group and rapamycin group detected by Western blot; B: the ratio of LC3-Ⅱ/LC3-Ⅰ in normal control group,miR-216b mimic group and rapamycin group (*: P<0.05)
图 2 Western blot检测miR-216b抑制细胞自噬水平Figure 2 Cell autophagy inhibited by miR-216b detected by Western blotA: the expression of Beclin-1,LC3-Ⅰ and LC3-Ⅱ in normal control group,miR-216b mimic group and rapamycin group detected by Western blot; B: the ratio of LC3-Ⅱ/LC3-Ⅰ in normal control group,miR-216b mimic group and rapamycin group (*: P<0.05)2.2 抑制miR-216b的表达能够促进宫颈癌细胞的自噬水平
转染miR-216b抑制剂的细胞中,miR-216b表达量出现明显下降,下降近80%。同时,其自噬点阳性细胞数明显增多,见图 3。通过Western blot检测发现,自噬相关蛋白Beclin-1和LC3-Ⅱ的表达均表现出增加,见图 4。结果表明,在HeLa细胞中,抑制miR-216b的表达能够促进自噬水平的升高。
![]() 图 3 荧光显微镜观察经miR-216b抑制剂转染处理后HeLa中GFP-LC3自噬点的分布变化Figure 3 Distribution of GFP-LC3 autophagy points in HeLa cells after transfection with miR-216b inhibitor observed by fluorescence microscopyA: the expression of miR-216b in anti-NC group and anti-miR-216b group after transfection with miR-216b inhibitors; B: the positive rate of autophagy points in anti-NC group and anti-miR-216b group after transfection with miR-216b inhibitor; C: the autophagy positive cells in anti-NC group and anti-miR-216b group observed by fluorescence microscope after transfection with miR-216b inhibitor. Bright green fluorescent spots represented autophagy positive cells
图 3 荧光显微镜观察经miR-216b抑制剂转染处理后HeLa中GFP-LC3自噬点的分布变化Figure 3 Distribution of GFP-LC3 autophagy points in HeLa cells after transfection with miR-216b inhibitor observed by fluorescence microscopyA: the expression of miR-216b in anti-NC group and anti-miR-216b group after transfection with miR-216b inhibitors; B: the positive rate of autophagy points in anti-NC group and anti-miR-216b group after transfection with miR-216b inhibitor; C: the autophagy positive cells in anti-NC group and anti-miR-216b group observed by fluorescence microscope after transfection with miR-216b inhibitor. Bright green fluorescent spots represented autophagy positive cells![]() 图 4 Western blot检测发现抑制miR-216b表达能够促进细胞自噬水平Figure 4 Inhibiting miR-216b expression could promote cells autophagy level observed by Western blotA: the expression of Beclin-1,LC3-Ⅰ,LC3-Ⅱ in anti-NC group and anti-miR-216b mimic group after transfection with miR-216b inhibitors detected by Western blot; B: the ratio of LC3-Ⅱ/LC3-Ⅰ in NC-anti group and anti-miR-216b mimic group after transfection with miR-216b inhibitors (*: P<0.05)
图 4 Western blot检测发现抑制miR-216b表达能够促进细胞自噬水平Figure 4 Inhibiting miR-216b expression could promote cells autophagy level observed by Western blotA: the expression of Beclin-1,LC3-Ⅰ,LC3-Ⅱ in anti-NC group and anti-miR-216b mimic group after transfection with miR-216b inhibitors detected by Western blot; B: the ratio of LC3-Ⅱ/LC3-Ⅰ in NC-anti group and anti-miR-216b mimic group after transfection with miR-216b inhibitors (*: P<0.05)2.3 miR-216b能够靶向结合Beclin-1
查找数据库发现,miR-216b能够与Beclin-1的3’UTR区域中的579-585段结合,见图 5结果显示。两种质粒分别与miR-216b mimic及其阴性对照共转染HeLa细胞,48 h后将细胞裂解,通过荧光素值检测发现,转染Beclin-1 3’UTR质粒载体(野生组)和miR-216b组,其荧光素值相对于对照组明显下降,而Beclin-1 3’UTR突变位点组(突变组)荧光素值相对于对照组,未出现明显变化,见图 6。 结果表明,miR-216b能够通过靶结合Beclin-1中的3‘UTR区域中的579~585段发挥其抑制作用。
3 讨论
MicroRNAs是真核生物细胞中一类长度约为17~24个核苷酸的内源性非编码小RNA。它本身不编码蛋白质,主要通过负性调控靶基因的表达发挥作用。近年来研究表明,某些miRNA的差异性表达与宫颈癌的发生、进展有密切关系,可以作为宫颈癌的诊断及预后的指标。宫颈癌中miRNA表达的失调与肿瘤的增殖、凋亡、侵袭和转移有重要关系[3-5]。
近年来miRNA在调节肿瘤自噬水平方面受到广泛关注,在维持细胞内环境稳定方面起着重要作用。本实验中,通过Western blot和GFP-LC3 shRNA转染细胞等检测方法,首次发现miR-216b能够显著降低HeLa细胞自噬水平,而施加miR-216b抑制剂后可明显提高自噬水平。本实验所选取的自噬相关蛋白-微管相关蛋白1轻链3(microtubule-associated protein 1 light chain 3,LC3)是目前研究较多的自噬标志性物质,其定位于前自噬泡和自噬泡膜表面,参与自噬体的形成,被认为是自噬特异性形成指标;Beclin-1是哺乳动物的自噬调控基因,是自噬发生的触发器,其表达的上调可诱导自噬的形成,可通过促进细胞凋亡诱导细胞自噬而抑制肿瘤的发展过程[12]。两种自噬指示蛋白在转染miR-216b mimic的作用下,均出现表达量降低。然而,转染miR-216b抑制剂后,自噬水平出现了上调,这与前述结果吻合,说明miR-216b的确能够抑制宫颈癌细胞的自噬水平。为进一步研究miR-216b在自噬过程中的功能,本研究通过荧光素酶报告基因的检测发现miR-216b能够靶结合Beclin-1的3’UTR区域,进而发挥其抑制作用。
Beclin-1是酵母菌ATG6在哺乳类动物的同源基因,是第一个被发现参与自噬过程的基因,其编码的Beclin-1蛋白可调控自噬前体的形成,引导相关蛋白定位于自噬体膜[13]。体外研究发现,Beclin-1缺失的小鼠自发和病毒诱导的肿瘤发生率增加[14-15],说明Beclin-1在抑制肿瘤发生方面有重要作用。本实验发现miR-216b能够负调控Beclin-1的表达来影响宫颈癌细胞自噬水平。
越来越多的研究发现机体细胞自噬活性变化对肿瘤的发生发展及对抗肿瘤药物的药效影响具有双重作用。研究发现自噬相关蛋白在宫颈癌中表达受抑制,下调自噬的形成,促进肿瘤的发生和发展[16],另外,也有研究发现,当自噬相关蛋白过表达后,宫颈癌细胞的生长会受到明显抑制[17],因此,通过调控自噬形成有可能成为未来宫颈癌治疗的策略之一,而本实验所研究的miR-216b的功能就是能够抑制宫颈癌细胞自噬的形成。宫颈癌是威胁女性健康的恶性肿瘤,目前,仍无有效的治疗方式。而自噬是维持细胞稳态的重要通路,在疾病中的作用逐步得到认同。本研究首次表明了miR-216b在宫颈癌自噬发生过程中的机制,为治疗宫颈癌提供了理论基础,也为开发类似药物提供了新的思路。
-
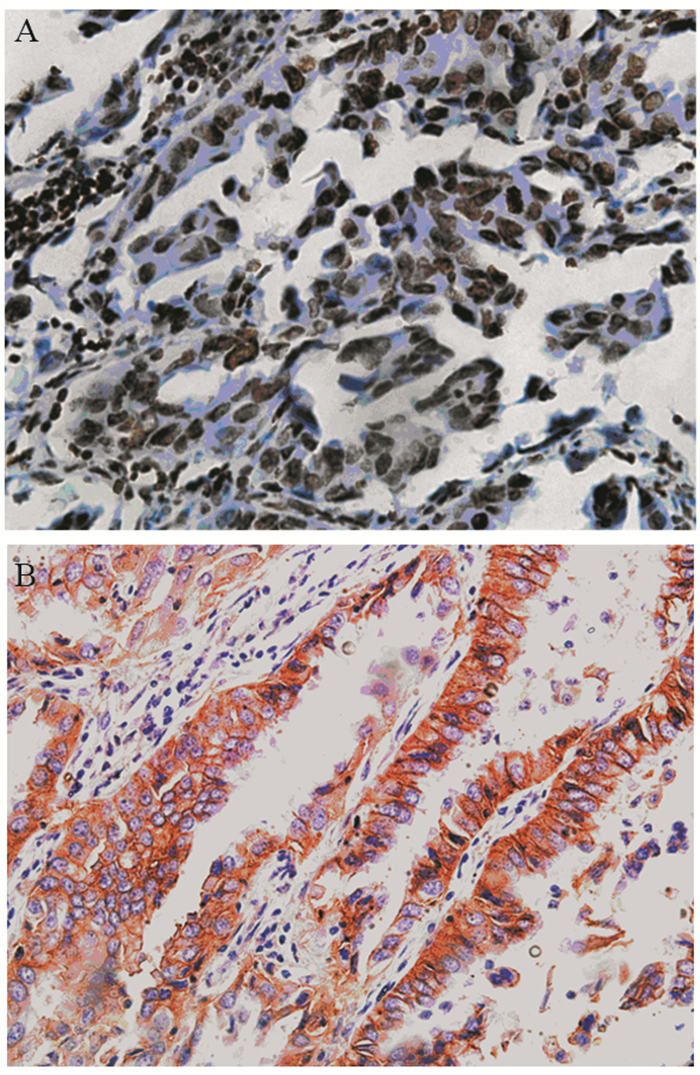
表 1 PTEN和CD147的表达与肺腺癌患者临床病理特征的关系
Table 1 Relationship between expression of PTEN and CD147 with clinicopathological characteristics of lung adenocarcinoma patients

表 2 肺腺癌组织中PTEN和CD147表达的相关分析 (n)
Table 2 Spearman analysis of PTEN and CD147 expression in lung adenocarcinoma tissues (n)

表 3 PTEN和CD147与肺腺癌患者EGFR基因突变的关系 (n)
Table 3 Spearman analysis of PTEN and CD147 expression with EGFR mutation in lung adenocarcinoma patients (n)

-
[1] Xu XY, Lin N, Li YM, et al.Expression of HAb18G/CD147 and its localization correlate with the progression and poor prognosis of non-small cell lung cancer[J].Pathol Res Pract, 2013, 209(6): 345-52. doi: 10.1016/j.prp.2013.02.015
[2] Tay Y, Kats L, Salmena L, et al.Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs[J].Cell, 2011, 147(2): 344-57. doi: 10.1016/j.cell.2011.09.029
[3] Chen Y, van de Vijver MJ, Hibshoosh H, et al.PTEN and NEDD4 in Human Breast Carcinoma[J].Pathol Oncol Res, 2016, 22(1): 41-7. doi: 10.1007/s12253-015-9971-2
[4] Shen H, Guan D, Shen J, et al.TGF-β1 induces erlotinib resistance in non-small cell lung cancer by down-regulating PTEN[J].Biomed Pharmacother, 2016, 77: 1-6. doi: 10.1016/j.biopha.2015.10.018
[5] Lotan TL, Wei W, Morais CL, et al.PTEN Loss as Determined by Clinical-grade Immunohistochemistry Assay Is Associated with Worse Recurrence-free Survival in Prostate Cancer[J].Eur Urol Focus, 2016, 2(2): 180-8. doi: 10.1016/j.euf.2015.07.005
[6] 梁育飞, 郑国启, 李春英, 等.COX-2、NF-κB、WT-1、PTEN在恶性腹膜间皮瘤中的表达及与预后的关系[J].肿瘤防治研究, 2016, 43(8):704-8. doi: 10.3971/j.issn.1000-8578.2016.08.011 Liang YF, Zheng GQ, Li CY, et al.Prognostic Significance of COX-2, NF-κB, WT-1 and PTEN Expression in Malignant Peritoneal Mesothelioma[J].Zhong Liu Fang Zhi Yan Jiu, 2016, 43(8): 704-8. doi: 10.3971/j.issn.1000-8578.2016.08.011
[7] Lee A, Rode A, Nicoll A, et al.Circulating CD147 predicts mortality in advanced hepatocellular carcinoma[J].J Gastroenterol Hepatol, 2016, 31(2): 459-66. doi: 10.1111/jgh.2016.31.issue-2
[8] Kaira K, Arakawa K, Shimizu K, et al.Relationship between CD147 and expression of amino acid transporters (LAT1 and ASCT2) in patients with pancreatic cancer[J].Am J Transl Res, 2015, 7(2): 356-63. http://www.ajtr.org/files/ajtr0003635.pdf
[9] Xu X, Liu S, Lei B, et al.Expression of HAb18G in non-small lung cancer and characterization of activation, migration, proliferation, and apoptosis in A549 cells following siRNA-induced downregulation of HAb18G[J].Mol Cell Biochem, 2013, 383(1-2): 1-11. doi: 10.1007/s11010-013-1722-7
[10] 许建民, 董涛, 田湘萍, 等.PTEN和AKT2及CD147在胃癌组织中的表达及意义[J].中国现代普通外科进展, 2010, 13(9): 681-4. http://www.cnki.com.cn/Article/CJFDTOTAL-PWJZ201009006.htm Xu JM, Dong T, Tian XP, et al.Expression of PTEN and CD147 and AKT2 in gastric carcinoma tissues and the clinical significance[J].Zhongguo Xian Dai Pu Tong Wai Ke Jin Zhan, 2010, 13(9): 681-4. http://www.cnki.com.cn/Article/CJFDTOTAL-PWJZ201009006.htm
[11] 李昌秀, 徐红梅, 杨达荣, 等.CD147PTEN E-钙黏蛋白在食管癌组织中表达与临床病理的关系[J].检验医学与临床, 2013, 10(2): 129-31. http://www.cnki.com.cn/Article/CJFDTOTAL-JYYL201302002.htm Li CX, Xu HM, Yang DR, et al.The expression of CDl47, PTEN and E-cadherin in esophagcal carcinoma and their correlation with clinical-pathological characteristics[J].Jian Yan Yi Xue Yu Lin Chuang, 2013, 10(2): 129-31. http://www.cnki.com.cn/Article/CJFDTOTAL-JYYL201302002.htm
[12] 徐小艳, 郅程, 陈清, 等.非小细胞肺癌组织中PTEN和HAb18G的表达及相关关系研究[J].实用医学杂志, 2012, 28(5): 701-4. http://www.cnki.com.cn/Article/CJFDTOTAL-SYYZ201205008.htm Xu XY, Zhi C, Chen Q, et al.Study on the correlation of PTEN and HAb18G expression in non small lung carcinoma tissues[J].Shi Yong Yi Xue Za Zhi, 2012, 28(5): 701-4. http://www.cnki.com.cn/Article/CJFDTOTAL-SYYZ201205008.htm
[13] 刘曙光, 邹振宁, 马红梅, 等.CD147和PTEN在非小细胞肺癌中的表达及与临床病理因素的相关性分析[J].重庆医学, 2016, 45(19): 2638-40, 2644. doi: 10.3969/j.issn.1671-8348.2016.19.015 Liu SG, Zou ZN, Ma HM, et al.The expression of CD147 and PTEN in non small cell lung cancer and their correlation with clinical pathological factors[J].Chongqing Yi Xue, 2016, 45(19): 2638-40, 2644. doi: 10.3969/j.issn.1671-8348.2016.19.015
[14] 顾学文, 陈月香, 田秀春, 等.PTEN抑癌基因在人胃癌组织中的表达及意义[J].肿瘤研究与临床, 2005, 17(1): 18-20. http://www.cnki.com.cn/Article/CJFDTOTAL-ZLYJ200501007.htm Gu XW, Chen YX, Tian XC, et al.Expression and significance of suppressor gene PTEN in human gastric cancer tissues[J].Zhong Liu Yan Jiu Yu Lin Chuang, 2005, 17(1): 18-20. http://www.cnki.com.cn/Article/CJFDTOTAL-ZLYJ200501007.htm
[15] 米登海, 易继林, 刘恩宇, 等.血管内皮生长因子与抑癌基因PTEN在肝细胞癌组织中表达及临床意义[J].中华肿瘤防治杂志, 2006, 13(15): 1158-62. doi: 10.3969/j.issn.1673-5269.2006.15.010 Mi DH, Yi JL, Liu EY, et al.Expressions of VEGF and PTEN in liver cancer and their clinical significance[J].Zhonghua Zhong Liu Fang Zhi Za Zhi, 2006, 13(15): 1158-62. doi: 10.3969/j.issn.1673-5269.2006.15.010
[16] Moon SK, Kim HM, Kim CH.PTEN induces G1, cell cycle arrest and inhibits MMP-9 expression via the regulation of NF-κB and AP-1 in vascular smooth muscle cells[J].Arch Biochem Biophys, 2004, 421(2): 267-76. doi: 10.1016/j.abb.2003.11.007
[17] Tian T, Nan KJ, Wang SH, et al.PTEN regulates angiogenesis and VEGF expression through phosphatase-dependent and-independent mechanisms in HepG2 cells[J].Carcinogenesis, 2010, 31(7): 1211-9. doi: 10.1093/carcin/bgq085
[18] Marchiq I, Albrengues J, Granja S, et al.Knock out of the BASIGIN/CD147 chaperone of lactate/H+symporters disproves its pro-tumour actionviaextracellular matrix metalloproteases (MMPs) induction[J].Oncotarget, 2015, 6(28): 24636-48. doi: 10.18632/oncotarget
[19] Su J, Chen X, Kanekura T.A CD147-targeting siRNA inhibits the proliferation, invasiveness, and VEGF production of human malignant melanoma cells by down-regulating glycolysis[J].Cancer Lett, 2009, 273(1): 140-7. doi: 10.1016/j.canlet.2008.07.034
[20] 贺锐, 李建民, 王跃华, 等.EGFR基因突变与肺腺癌新分类及临床病理特征的关系[J].临床与实验病理学杂志, 2013, 29(12): 1323-8. http://cdmd.cnki.com.cn/Article/CDMD-10114-1014318506.htm He R, Li JM, Wang YH, et al.Correlation of epidermal growth factor receptor mutations with the new lung adenocarcinoma classification and their clinicopathological features[J].Lin Chuang Yu Shi Yan Bing Li Xue Za Zhi, 2013, 29(12): 1323-8. http://cdmd.cnki.com.cn/Article/CDMD-10114-1014318506.htm
[21] Hong SJ, Kim TJ, Choi YW, et al.Radiogenomic correlation in lung adenocarcinoma with epidermal growth factor receptor mutations: Imaging features and histological subtypes[J].Eur Radiol, 2016, 26(10): 3660-8. doi: 10.1007/s00330-015-4196-z




 下载:
下载: