Expression and Clinical Significance of Forkhead Box M1 and Polo-like Kinase 1 in Breast Carcinoma Tissues
-
摘要:目的
探讨叉头框转录因子M1(FOXM1)及Polo样激酶1(PLK1)在乳腺癌组织中的表达及临床意义。
方法采用免疫组织化学法检测FOXM1及PLK1蛋白在803例乳腺浸润性导管癌和乳腺癌旁组织中的表达情况及其与乳腺癌组织临床病理特征间的关系。
结果FOXM1及PLK1在乳腺浸润性导管癌组织中的阳性表达率分别为59.78%(480/803)和27.90%(224/803),显著高于其在乳腺癌旁组织中的表达(29.89%, 0),差异具有统计学意义(χ2=145.011, χ2=260.307, P=0.000)。FOXM1及PLK1蛋白的表达与乳腺癌的组织学分级、淋巴结转移及临床分期相关,而与肿瘤大小无关,且二者表达具有正相关关系(r=0.414, P < 0.01)。
结论FOXM1及PLK-1可能协同参与了乳腺癌的发生、发展,并可能成为乳腺癌预后评估的重要指标。
-
关键词:
- 乳腺癌 /
- 叉头框转录因子M1 /
- Polo-like激酶1
Abstract:ObjectiveTo investigate the expression and clinical significance of forkhead box M1 (FOXM1) and polo-like kinase 1 (PLK1) in breast carcinoma tissues.
MethodsImmunohistochemical staining was used to detect the expression of FOXM1 and PLK1 in 803 cases of invasive ductal breast carcinoma and para-tumor breast tissues, and we evaluated the relationship of FOXM1 and PLK1 expression with the clinical pathological features of invasive ductal breast carcinoma patients, including histological grade, lymph node metastasis and pTNM stage.
ResultsThe positive expression rates of FOXM1 and PLK1 protein in invasive ductal breast carcinoma tissues were 59.78% (480/803) and 27.90% (224/803), significantly higher than those in para-tumor breast tissues (29.89%/0)(χ2=145.011, χ2=260.307, P=0.000). FOXM1 and PLK1 expression were significantly related with the histopathologic grade, lymph node metastasis and pTNM stage, while not correlated with the tumor size. There was a positive relation between the expression of FOXM1 and PLK1 (r=0.414, P < 0.01).
ConclusionFOXM1 and PLK1 may be involved in the breast carcinogenesis and progression synergistically, and they might play important roles in the prognosis evaluation of breast carcinoma.
-
Key words:
- Breast carcinoma /
- FOXM1 /
- PLK-1
-
0 引言
乳腺癌是女性最常见的恶性肿瘤之一,严重威胁着女性的健康和生命。乳腺癌的发生发展机制、生物学行为、预后评估指标及寻找特异性靶向治疗方法等一直是医学界广泛关注的焦点。叉头框转录因子M1(forkhead box M1, FOXM1)是细胞周期的重要调控因子,促进了多种肿瘤的形成、发展、浸润和转移,是抗肿瘤的潜在靶点。Polo-like激酶1(polo-like kinase 1, PLK1)是一种高度保守的丝/苏氨酸蛋白激酶,参与了DNA复制、转录、细胞内信号转导、损伤修复、P53调节等多个环节,而且和肿瘤的侵袭、转移以及多药耐药有一定的关系。本研究通过免疫组织化学的方法检测FOXM1和PLK1蛋白在乳腺浸润性导管癌及癌旁乳腺组织中的表达情况,并分析二者表达与乳腺癌临床病理特征间的关系,为临床治疗和预后的评估提供参考和理论依据。
1 资料与方法
1.1 标本来源
收集2005年1月13日至2014年10月31日河北省保定市第一中心医院病理科的乳腺浸润性导管癌存档石蜡切片,选取有完整临床病理资料的803例,乳腺癌旁组织(距肿瘤边缘5 cm的乳腺组织)作为对照。所有乳腺癌患者均为女性,年龄24~79岁,平均年龄51.45岁。组织学分级Ⅰ级164例、Ⅱ级361例、Ⅲ级278例。455例无淋巴结转移、348例有淋巴结转移;Ⅰ~Ⅱ期678例、Ⅲ~Ⅳ期125例。所有乳腺癌患者术前均未行放疗、化疗及内分泌治疗。
1.2 试剂与方法
兔抗人FOXM1多克隆抗体、兔抗人PLK1单克隆抗体均购自美国Abcam公司(稀释浓度1:150)。即用型快捷免疫组织化学MaxVisionTM检测试剂盒和氨基联苯胺显色试剂盒均购自福州迈新生物生物科技有限公司。标本经10%中性福尔马林固定、石蜡包埋、4 μm厚连续切片、采用免疫组织化学MaxVision两步法染色(具体操作步骤参照试剂盒说明书进行),以PBS代替一抗作为阴性对照,以已知FOXM1和PLK1阳性的胃腺癌组织作为阳性对照。
1.3 免疫组织化学染色结果判定
FOXM1和PLK1蛋白的阳性表达为棕黄色颗粒,位于细胞核和细胞质。采取双盲法阅片,结合阳性细胞比例和阳性细胞着色强度进行判定。FOXM1:(1)染色强度,不着色为0分,呈淡黄色为1分,黄色为2分,棕褐色为3分;(2)阳性染色细胞百分比 < 1%为0分,≥1%~10%为1分,11%~50%为2分, > 50%为3分。将两项评分的结果相乘,≥4分为阳性表达,0~3分为阴性表达[1]。PLK1:(1)染色强度,不着色为0分,呈淡黄色为1分,黄色为2分,棕褐色为3分;(2)阳性染色细胞百分比0~4%为1分,5%~20%为2分,21%~40%为3分,41%~60%为4分,61%~80%为5分,81%~100%为6分,将两项评分的结果相乘,4~18分为阳性表达,0~3分为阴性表达[2]。
1.4 统计学方法
采用SPSS23.0统计学软件进行数据分析,计数资料采用卡方检验,多个样本率的两两比较采用卡方分割法,检验水准α=0.05。
2 结果
2.1 FOXM1和PLK1在各组的表达情况
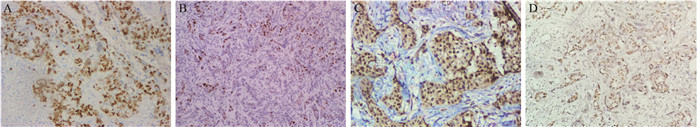
FOXM1及PLK1在乳腺浸润性导管癌组中的阳性表达率分别为59.78%(480/803)和27.90%(224/803),显著高于二者在乳腺癌旁组织中的表达[29.89%(240/803)和0(0/803)],差异具有统计学意义(χ2=145.011, χ2=260.307,P=0.000),见图 1,表 1。
![]() 图 1 乳腺癌组织FOXM1及PLK1的染色结果Figure 1 Expression of FOM1 and PLK1 in breast carcinoma tissuesA, C: strong positive expression of FOXM1 and PLK1 in breast carcinoma tissues, mainly in the cytoplasm and nucleus (IHC ×200); B, D: weakly positive expression of FOXM1 and PLK1 in breast carcinoma tissues (IHC ×100)表 1 FOXM1和PLK1在乳腺浸润性导管癌和癌旁组织中的表达[n(%)]Table 1 Expression of FOXM1 and PLK1 in invasive ductal breast carcinoma and para-tumor breast tissues [n(%)]
图 1 乳腺癌组织FOXM1及PLK1的染色结果Figure 1 Expression of FOM1 and PLK1 in breast carcinoma tissuesA, C: strong positive expression of FOXM1 and PLK1 in breast carcinoma tissues, mainly in the cytoplasm and nucleus (IHC ×200); B, D: weakly positive expression of FOXM1 and PLK1 in breast carcinoma tissues (IHC ×100)表 1 FOXM1和PLK1在乳腺浸润性导管癌和癌旁组织中的表达[n(%)]Table 1 Expression of FOXM1 and PLK1 in invasive ductal breast carcinoma and para-tumor breast tissues [n(%)]
2.2 FOXM1和PLK1蛋白与乳腺癌病理特征间的关系
FOXM1和PLK1蛋白的表达与乳腺癌组织学分级、淋巴结转移及临床分期密切相关,而与肿瘤大小无关(χ2=1.369/3.930, P > 0.05)。组织学Ⅲ级的乳腺癌组织中,FOXM1和PLK1蛋白的阳性表达率显著高于Ⅰ级和Ⅱ级乳腺癌,差异有统计学意义(与组织学Ⅰ级相比:χ2=20.381, P=0.000; χ2=10.136, P=0.001;与组织学Ⅱ级相比:χ2=20.144, P=0.000; χ2=26.027, P=0.000);但组织学Ⅰ级和Ⅱ级的乳腺癌组织相比,FOXM1和PLK1蛋白的表达差异无统计学意义(χ2=0.711, P=0.399; χ2=0.861, P=0.353)。FOXM1蛋白的表达与ER无相关性,而与HER2有正相关关系(r=0.130, P < 0.001)。PLK1蛋白的表达与ER呈负相关关系(r=-0.294, P < 0.001),而与HER2无相关性,见表 2。
表 2 FOXM1和PLK1与乳腺癌临床病理特征的关系Table 2 Correlation between FOXM1, PLK1 expression and clinicopathologic parameters of breast carcinoma patients
2.3 FOMX1和PLK1蛋白在乳腺浸润性导管癌中表达的相关性分析
Spearman相关分析结果显示:FOXM1和PLK1在乳腺浸润性导管癌中的表达呈正相关关系(r=0.414, P < 0.01),见表 3。
表 3 乳腺癌中FOXM1和PLK1表达的相关性Table 3 Relationship between expression of FOXM1 and PLK1
3 讨论
肿瘤的生成涉及很多复杂的环节,主要表现为细胞分裂及增殖的失调控。FOXM1是Forkhead蛋白家族中的一员,能够调节细胞周期的进展、调节与纺锤体装配及染色体分离相关基因的表达,维持基因组的稳定性[3]。研究发现FOXM1在多种实体肿瘤中高表达,而且与肿瘤的不良预后有关[4-6]。FOXM1的异常表达促进肿瘤细胞增殖,增强抗凋亡能力[6];参与上皮间质转化、调节肿瘤细胞运动、参与侵袭及转移前靶器官小环境的形成;活化Akt-Snail信号途径,促进肿瘤细胞的转移[7]。本研究结果显示,与乳腺癌旁组织相比,乳腺浸润性导管癌中FOXM1蛋白的阳性表达率显著升高,两组差异具有统计学意义(P < 0.05),而且FOXM1的表达与乳腺浸润性导管癌的组织学分级、淋巴结转移和pTNM分期相关,进一步证实FOXM1是一个强有力的致癌因子,可能参与了乳腺癌的发生,而且与乳腺癌浸润、转移的能力有关,可能成为乳腺癌预后评估重要的指标。Millour等发现FOXM1可直接与ERα的启动子连接,激活ERα转录,通过诱导ERα蛋白的表达,促进乳腺癌细胞的增殖。同时二者形成正反馈,还能够增强雌激素的促有丝分裂作用,在乳腺癌的生成过程中发挥重要作用[8]。Francis等证实,FOXM1是HER-2下游的靶基因,HER-2可能通过调节FOXM1的表达发挥促进乳腺肿瘤形成的作用[9]。本实验结果显示,FOXM1与ER表达无关,而与HER-2的表达呈正相关关系,与既往研究结果的差异可能与病例选择及实验方法不同有关,仍需进一步探讨。
PLK1是Polo-like激酶家族的重要成员,控制着有丝分裂的多个阶段[10],在G1/S期转换和DNA复制方面具有细胞特异性[11-12]。PLK1在多种肿瘤中高表达,并与肿瘤预后有关[2, 13-15]。本研究发现PLK1在乳腺浸润性导管癌中的阳性表达率显著高于乳腺癌旁组织,提示PLK1过表达可能通过使细胞周期调控异常,出现不成熟中心体的分离及染色体的不稳定,导致非整倍体的出现及肿瘤的恶性转化。而且PLK1与乳腺癌的组织学分级、淋巴结转移及pTNM分期相关,提示PLK1与乳腺癌组织的侵袭及浸润、转移能力有关,可能成为评估乳腺癌患者预后的重要指标。Maire等研究发现,与乳腺癌的LA型、LB型、HER-2过表达型相比,PLK1在TNBC中表达水平更高,而且认为PLK1是TNBC潜在的治疗靶点[16]。King等也证实PLK1与ER-α的表达相关,并与TNBC关系更密切[2]。本研究显示PLK1与ER表达呈负相关关系,提示PLK1过表达可能在ER阴性乳腺癌的癌变过程中发挥更重要的作用。
FOXM1和PLK1之间有复杂的内部调节关系,成为细胞周期控制的关键点。FOXM1通过周期性上调CCNB1和PLK1在内的一组基因的表达控制着有丝分裂的启动,而PLK1通过直接的磷酸化作用,调节FOXM1的转录,并增强其活性,二者参与形成一个激酶诱导的正反馈链[17-18]。本研究结果显示FOMX1和PLK1蛋白的表达具有正相关关系,进一步证实二者在乳腺癌癌变及进展中发挥了协同作用。
综上所述,FOXM1及PLK1蛋白在乳腺癌中高表达,并与乳腺癌侵袭、转移能力有关,二者可能成为乳腺癌新的预后评估指标及潜在的治疗靶点,为乳腺癌个体化治疗提供了新的思路。
-
表 1 FOXM1和PLK1在乳腺浸润性导管癌和癌旁组织中的表达[n(%)]
Table 1 Expression of FOXM1 and PLK1 in invasive ductal breast carcinoma and para-tumor breast tissues [n(%)]

表 2 FOXM1和PLK1与乳腺癌临床病理特征的关系
Table 2 Correlation between FOXM1, PLK1 expression and clinicopathologic parameters of breast carcinoma patients

表 3 乳腺癌中FOXM1和PLK1表达的相关性
Table 3 Relationship between expression of FOXM1 and PLK1

-
[1] 刘怡茜, 郭人花, 刘连科, 等.叉头框转录因子M1在非小细胞癌中的表达及其与患者临床病理特征和生存的关系[J].中华肿瘤杂志, 2011, 33(6): 426-30. Liu YQ, Guo RH, Liu LK, et al. Correlation between expression of forkhead box M1(FOXM1) and clinicopathological features and prognosis in patients with non-small cell lung cancer (NSCLC)[J]. Zhonghua Zhong Liu Za Zhi, 2011, 33(6): 426-30.
[2] King SI, Purdie CA, Bray SE, et al. Immunohistochemical detection of Polo-like kinase-1(PLK1) in primary breast cancer is associated with TP53 mutation and poor clinical outcome[J]. Breast Cancer Res, 2012, 14(2): R40. doi: 10.1186/bcr3136
[3] Laoukili J, Stahl M, Medema RH. FoxM1: at the crossroads of ageing and cancer[J]. Biochim Biophys Acta, 2007, 1775(1): 92-102. https://www.researchgate.net/publication/6780074_FoxM1_At_the_crossroads_of_ageing_and_cancer
[4] Pilarsky C, Wenzig M, Specht T, et al. Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data[J]. Neoplasia, 2004, 6(6): 744-50. doi: 10.1593/neo.04277
[5] Chan DW, Yu SY, Chiu PM, et al. Over-expression of FOXM1 transcription factor is associated with cervical cancer progression and pathogenesis[J]. J Pathol, 2008, 215(3): 245-52. doi: 10.1002/(ISSN)1096-9896
[6] Halasi M, Gartel AL. FOX (M1) news-it is cancer[J]. Mol Cancer Ther, 2013, 12(3): 245-54. doi: 10.1158/1535-7163.MCT-12-0712
[7] Park HJ, Gusarova G, Wang Z, et al. Deregulation of FoxM1b leads to tumour metastasis[J]. EMBO Mol Med, 2011, 3(1): 21-34. doi: 10.1002/emmm.201000107
[8] Millour J, Constantinidou D, Stavropoulou AV, et al. FOXM1 is a transcriptional target of ERalpha and has a critical role in breast cancer endocrine sensitivity and resistance[J]. Oncogene, 2010, 29(20): 2983-95. doi: 10.1038/onc.2010.47
[9] Francis RE, Myatt SS, Krol J, et al. FoxM1 is a downstream target and marker of HER2 overexpression in breast cancer[J]. Int J Oncol, 2009, 35(1): 57-68.
[10] Wang G, Chen Q, Zhang X, et al. PCM1 recruits PLK1 to the pericentriolar matrix to promote primary cilia disassembly before mitotic entry[J]. J Cell Sci, 2013, 126(Pt6): 1355-65. https://www.researchgate.net/profile/Gang_Wang67/publication/235367959_PCM1_Recruits_Plk1_to_Pericentriolar_Matrix_to_Promote_Primary_Cilia_Disassembly_before_Mitotic_Entry/links/5402c0f80cf2bba34c1b989b.pdf?inViewer=true&disableCoverPage=true&origin=publication_detail
[11] Factor VM, Seo D, Ishikawa T, et al. Loss of c-Met disrupts gene expression program required for G2/M progression during liver regeneration in mice[J]. PLoS One, 2010, 5(9): pⅱ:e12739.
[12] Zhang Z, Zhang G, Kong C. FOXM1 participates in PLK1-regulated cell cycle progression in renal cell cancer cells[J]. Oncol Lett, 2016, 11(4): 2685-91. https://www.researchgate.net/publication/294730345_FOXM1_participates_in_PLK1-regulated_cell_cycle_progression_in_renal_cell_cancer_cells
[13] Liu XS, Song B, Elzey BD, et al. Polo-like kinase 1 facilitates loss of Pten tumor suppressor-induced prostate cancer formation[J]. J Biol Chem, 2011, 286(41): 35795-800. doi: 10.1074/jbc.C111.269050
[14] Song B, Liu XS, Rice SJ, et al. PLK1 phosphorylation of orc2 and hbo1 contributes to gemcitabine resistance in pancreatic cancer[J]. Mol Cancer Ther, 2013, 12(1): 58-68. doi: 10.1158/1535-7163.MCT-12-0632
[15] Zhang G, Zhang Z, Liu Z. Polo-like kinase 1 is overexpressed in renal cancer and participates in the proliferation and invasion of renal cancer cells[J]. Tumour Biol, 2013, 34(3): 1887-94. doi: 10.1007/s13277-013-0732-0
[16] Maire V, Némati F, Richardson M, et al. Polo-like kinase 1: a potential therapeutic option in combination with conventional chemotherapy for the management of patients with triple-negative breast cancer[J]. Cancer Res, 2013, 73(2): 813-23. doi: 10.1158/0008-5472.CAN-12-2633
[17] Myatt SS, Kongsema M, Man CW, et al. SUMOylation inhibits FOXM1 activity and delays mitotic transition[J]. Oncogene, 2014, 33(34): 4316-29. doi: 10.1038/onc.2013.546
[18] Fu Z, Malureanu L, Huang J, et al. Plk1-dependent phosphorylation of FOXM1 regulates a transcriptional programme required for mitotic progression[J]. Nat Cell Biol, 2008, 10(9): 1076-82. doi: 10.1038/ncb1767



 下载:
下载:

