Effect of Ectopic Expression of Transmembrane Adaptor PAG1 on Motility of Cutaneous Squamous Cell Carcinoma A431 Cells in vitro
-
摘要:目的
研究跨膜接头蛋白PAG1过表达对人皮肤鳞状细胞癌A431运动能力的影响。
方法构建PAG1-EGFP融合蛋白的慢病毒表达载体,采用反转录病毒转染的方法建立PAG1过表达的A431细胞株,实验分为3组:亲本细胞组(未进行基因转染的A431细胞)、对照组(A431细胞转染仅含EGFP阴性对照病毒)、实验组(A431细胞转染PAG1-EGFP病毒)。流式细胞术检测细胞转染率,实时荧光定量PCR及Western blot检测转染后PAG1 mRNA及其蛋白表达,进一步验证细胞转染成功与否;利用划痕修复实验、Transwell迁移实验、侵袭实验三种不同的方法检测PAG1基因的过表达是否会对皮肤鳞癌细胞的生长、迁移和侵袭能力产生影响。
结果实验组和对照组目的基因病毒的转染表达率分别为(94.97±0.15)%、(94.60±0.35)%;实验组细胞中PAG1基因mRNA的表达量约为亲本细胞组的1.6倍(P=0.000),转染后实验组中PAG1蛋白的相对表达水平明显增加(P=0.000),建立了稳定过表达PAG1的A431细胞系;PAG1过表达明显降低A431细胞愈合率(P=0.000);实验组A431细胞迁移、侵袭能力明显降低(P=0.001, P=0.000)。
结论PAG1的过表达能抑制人皮肤鳞癌细胞运动能力,影响肿瘤的浸润及远处转移。
Abstract:ObjectiveTo investigate the effect of the ectopic expression of transmembrane protein PAG1 on the motility of human cutaneous squamous cell carcinoma A431 cells.
MethodsThe PAG1-EGFP lentiviral vector was constructed. A431 cells were transfected with PAG1-EGFP lentiviral vector (Experimental group) or negative-EGFP lenti-viral vector (Control group), or remained untransfected(Parental group). The transfection efficiency was detected by flow cytometry. To further verify and determine the success of the infection, the expression of PAG1 mRNA and protein were detected by real-time fluorescence quantitative PCR and Western blot, respectively. After additional culture, the wound-healing assay and Transwell chamber assay were performed to evaluate the abilities of migration and invasion.
ResultsThe transfection efficiency in the experimental group and control group were (94.97±0.15)% and (94.60±0.35)%, respectively. After transfected with PAG1-EGFP vector, the expression of PAG1 mRNA was about 1.6 times enhanced than that of untrasfected cells(P=0.000). The PAG1 protein levels were significantly increased after transfection with PAG1-EGFP lentiviral vector (P=0.000). The A431 cell line with PAG1 overexpression was successfully constructed; the ectopic expression of PAG1 significantly decreased the cells healing rate(P=0.000). Moreover, cell migration and invasion abilities were significantly inhibited(P=0.001, P=0.000).
ConclusionThe elevated levels of PAG1 could obviously inhibit the mobility of human cutaneous squamous cell carcinoma cells in vitro, which suggesting that the overexpression of PAG1 may influence their metastatic ability in vivo.
-
Key words:
- Cutaneous squamous cell carcinoma /
- PAG1 /
- Migration /
- Invasion
-
0 引言
皮肤鳞状细胞癌(cutaneous squamous cell carcinoma,cSCC)起源于表皮角质形成细胞,是世界第二大最常见的非黑色素瘤皮肤癌[1]。肿瘤复发和远处转移是高危型皮肤鳞状细胞癌患者不良预后的主要原因,因此控制肿瘤转移是目前高危型皮肤鳞状细胞癌研究的重点之一。肿瘤转移涉及很多基因的改变和作用。跨膜接头蛋白PAG1(phosphoprotein associated with glycosphingolipid microdomains 1)是近些年新发现的Src家族成员,主要参与细胞的生长、转化等过程,并与许多肿瘤的发生、发展相关[2]。本研究通过基因转染PAG1,观察PAG1过表达对人皮肤鳞状细胞癌A431细胞运动能力的影响,探讨PAG1与肿瘤转移相关的机制。
1 材料与方法
1.1 主要材料
人皮肤鳞癌细胞株A431购自中国科学院上海生命科学院细胞资源中心,PAG1-EGFP慢病毒融合蛋白表达载体由上海吉凯公司构建。PAG1引物由南京金斯瑞生物科技有限公司合成,反转录及实时荧光定量试剂盒均购自美国Roch公司。Transwell小室(8.0 μm孔径PC膜)购自美国Corning公司,Matrigel基质胶购自美国BD公司。流式细胞仪购自美国BD公司。PCR仪购自美国Bio-Rad公司。
1.2 方法
1.2.1 PAG1过表达细胞系制备
根据慢病毒载体操作说明,收集处于对数生长期的正常A431细胞,以5 000个每孔接种于96孔板,按MOI值为100加入反转录病毒液。扩大培养至6孔板后,收集部分转染的细胞,流式细胞术(FCM)检测表达绿色荧光蛋白的细胞比例,计算转染效率,剩余细胞继续扩大培养建立稳定的PAG1基因过表达细胞系。本实验共分为三组:(1)亲本细胞组(未进行基因转染的A431细胞);(2)对照组(A431细胞转染仅含EGFP阴性对照病毒);(3)实验组(A431细胞转染PAG1-EGFP病毒)。
1.2.2 实时荧光定量PCR
TRIzol提取细胞总RNA。SYBR Green标记产物,按照荧光定量PCR检测试剂盒操作说明建立反应体系。PAG1基因上游引物:5’-TCAGCCTGAGAGGAGGAAAT-3’,下游引物:5’-GCTCCTGCTACTTGGGAGTC-3’;GAPDH基因上游引物:5’-GATGACCTTGCCCACAGCCT-3’,下游引物:5’-ATCTCTGCCCCCTCTGCTGA-3’。反应条件:95℃ 10 min;95℃ 10 s、60℃ 20 s、72℃ 10 s,40个循环。各样品目的基因和管家基因进行实时荧光定量PCR反应,分别获得Ct值,通过2-△△Ct方法分析各样品相对PAG1表达差异,每组实验重复3次。各组实验以亲本细胞组的表达量设定为1,计算出其他组的相对表达量,以相对倍数进行作图。
1.2.3 Western blot 检测
收集处于对数生长期的三组细胞,每组约1×107细胞,加入200 μl预冷的蛋白裂解液冰上裂解2 h,4℃、14 000 r/min离心20 min,收集上清液。采用BCA法检测蛋白浓度,加入5×SDS上样缓冲液,100℃加热5 min。10% SDS-PAGE电泳后转膜,5%脱脂奶粉室温摇床封闭2 h,加入一抗4℃缓慢摇床过夜孵育。TBST漂洗3次,每次15 min,加入辣根过氧化物酶标记二抗,室温低速摇床孵育2 h。加入ECL显色液,室温避光孵育1~3 min,曝光、显影。
1.2.4 划痕修复实验
分别收集处于对数生长期的各组细胞,以1×106每孔接种于6孔板,置于37℃、5%CO2培养箱中培养直至细胞融合度约为95%,用20 μl微量移液头在6孔板内垂直线性划痕,D-hanks液冲洗去除飘落细胞后加入无血清培养液继续培养,划痕后0、36 h取样,相差显微镜下随机选择3个100×视野拍照,比较各组间划痕愈合差异,用愈合率代表细胞迁移愈合能力。愈合率=(愈合前细胞间空白区域面积-愈合后细胞间空白区域面积)/愈合前细胞间空白区域面积×100%。
1.2.5 Transwell细胞迁移及侵袭实验
(1) 迁移实验:三组细胞用无血清培养液饥饿12 h,用含0.5%FBS的G-DMEM培养液制备单细胞悬液,调整细胞浓度为2×105每毫升,Transwell小室上室分别加入各组细胞的单细胞悬液200 μl(4×104细胞),而小室下室加入650 μl含10%FBS的G-DMEM培养液,置于37℃、5%CO2培养箱中培养12 h。取出小室,PBS淋洗后,置于甲醇溶液中,室温固定30 min,再用0.1%结晶紫染色20 min,用棉签轻轻擦去微孔膜上层的细胞。在倒置光学显微镜下观察并采集图像,随机选取4个视野,计数穿过微孔膜的细胞,并计算平均值。实验重复3次。
(2) 细胞侵袭实验:将50 mg/ml的Matrigel基质胶4℃过夜融化,用无血清的G-DMEM培养液将其1:8稀释混匀。100 μl包被Transwell小室聚碳酸脂膜上室面,37℃过夜孵育凝固备用。整个操作过程在冰上及无菌条件下进行。调整细胞浓度为1×106每毫升,加入小室后继续孵育24 h,其他实验步骤同迁移实验。
1.3 统计学方法
采用SPSS17.0统计软件分析实验数据,结果以(x±s)的形式表示,各组间数据的两两比较采用t检验,P<0.05为差异有统计学意义。
2 结果
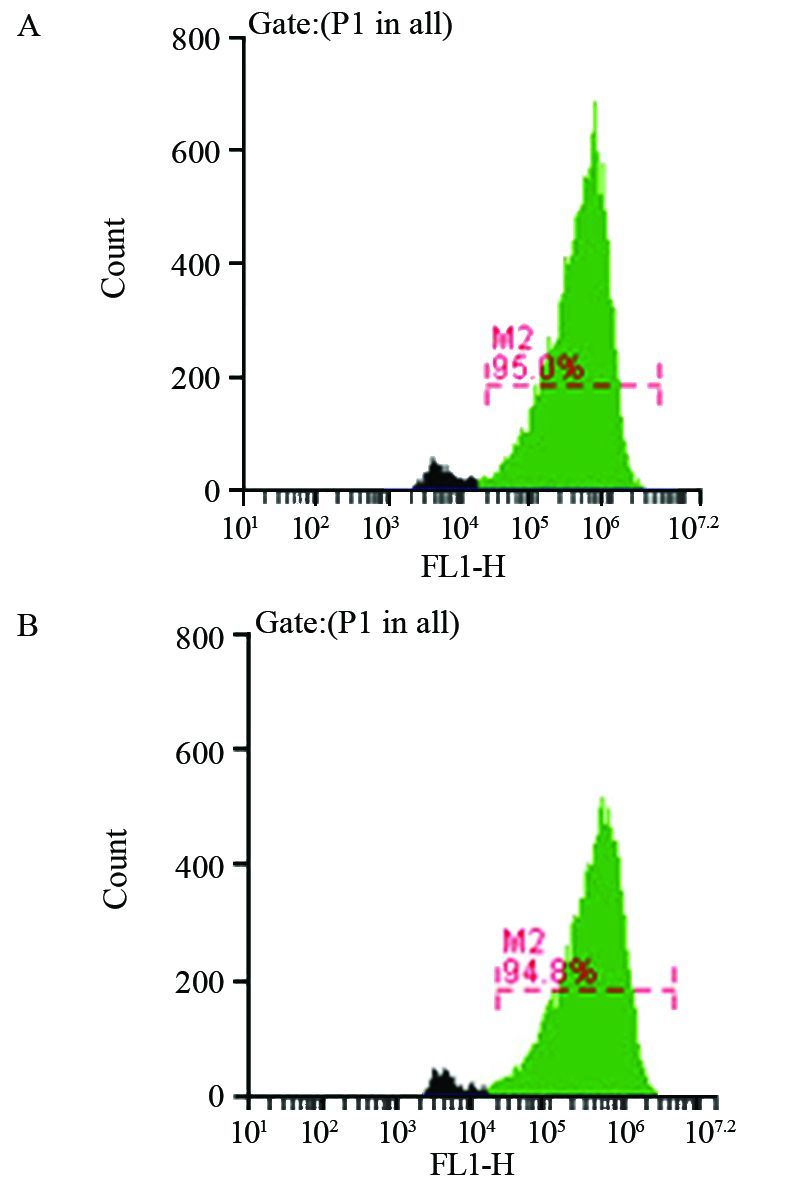
2.1 反转录病毒的转染效率
当MOI为100时,FCM检测反转录病毒感染A431细胞效率,见图 1,实验组和对照组分别为(94.97±0.15)%、(94.60±0.35)%,证实转染成功。
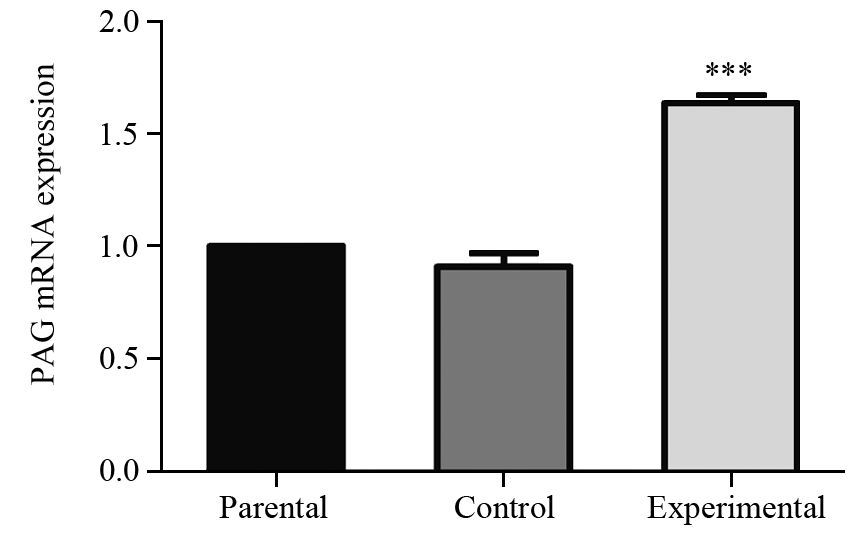
2.2 转染后PAG1基因mRNA表达的改变
实验组细胞中PAG1基因mRNA的表达量为亲本细胞组的1.6倍,见图 2,统计结果显示,实验组和亲本细胞组PAG1基因mRNA表达差异有统计学意义(P=0.000),对照组和亲本细胞组表达差异无统计学意义(P=0.070)。
![]() 图 2 实时荧光定量PCR分析转染后PAG1 mRNA的表达Figure 2 Expression level of PAG1 mRNA assayed by Real-time fluorescence quantitative PCR after transfectionParental: A431 cells were not transfected; Control: A431 cells were transfected with neg-EGFP; Experimental: A431 cells were transfected with PAG1-EGFP; ***: P<0.001,compared with control group
图 2 实时荧光定量PCR分析转染后PAG1 mRNA的表达Figure 2 Expression level of PAG1 mRNA assayed by Real-time fluorescence quantitative PCR after transfectionParental: A431 cells were not transfected; Control: A431 cells were transfected with neg-EGFP; Experimental: A431 cells were transfected with PAG1-EGFP; ***: P<0.001,compared with control group2.3 Western blot检测PAG1蛋白表达水平
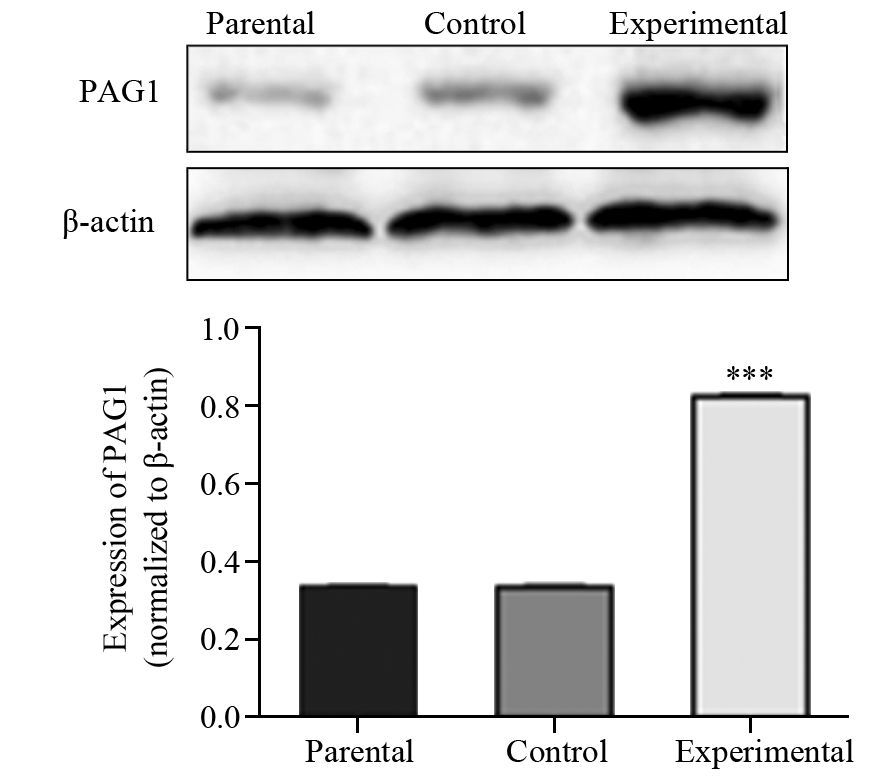
Western blot结果显示,与对照组和亲本细胞组相比,转染后实验组PAG1蛋白的相对表达水平明显增加(P=0.000),亲本细胞组与对照组间表达差异无统计学意义(P=0.888),见图 3,这与PAG1在mRNA水平的表达结果相一致。进一步证实带有报告基因EGFP的PAG1成功转染A431细胞,建立了稳定过表达PAG1的皮肤鳞癌细胞系。
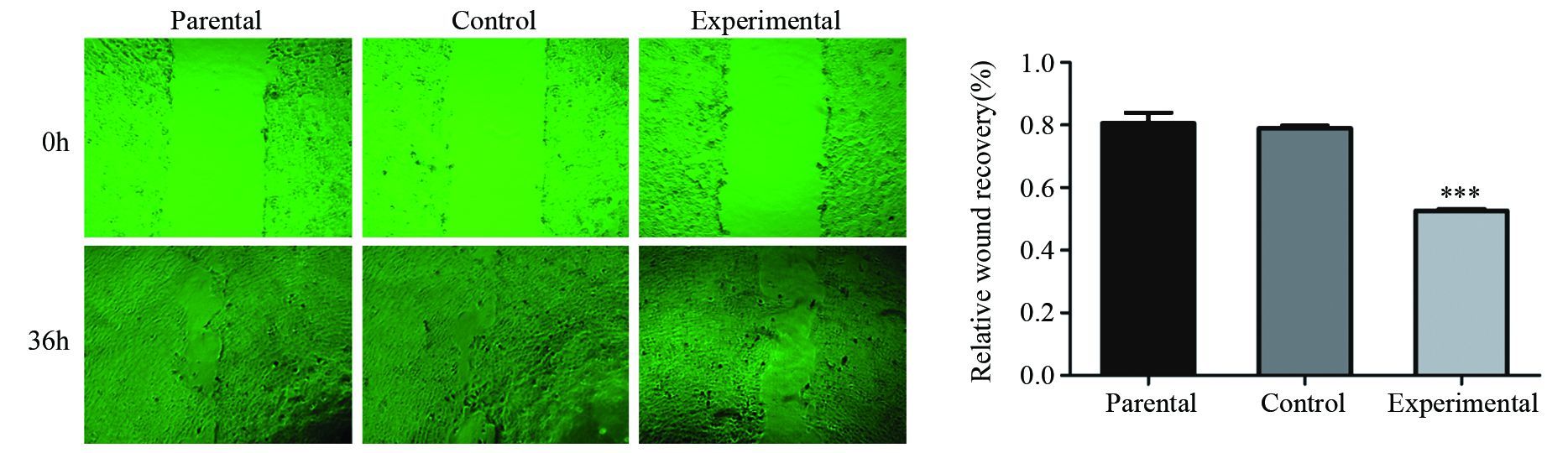
2.4 划痕修复实验检测PAG1过表达对A431细胞愈合率的影响
细胞划痕修复实验结果显示,划痕36 h后,各组间细胞均有一定程度愈合,其中实验组与对照组和亲本细胞组相比,划痕愈合率明显降低,差异有统计学意义(P=0.000),而亲本细胞组和对照组之间差异无统计学意义(P=0.624),见图 4。
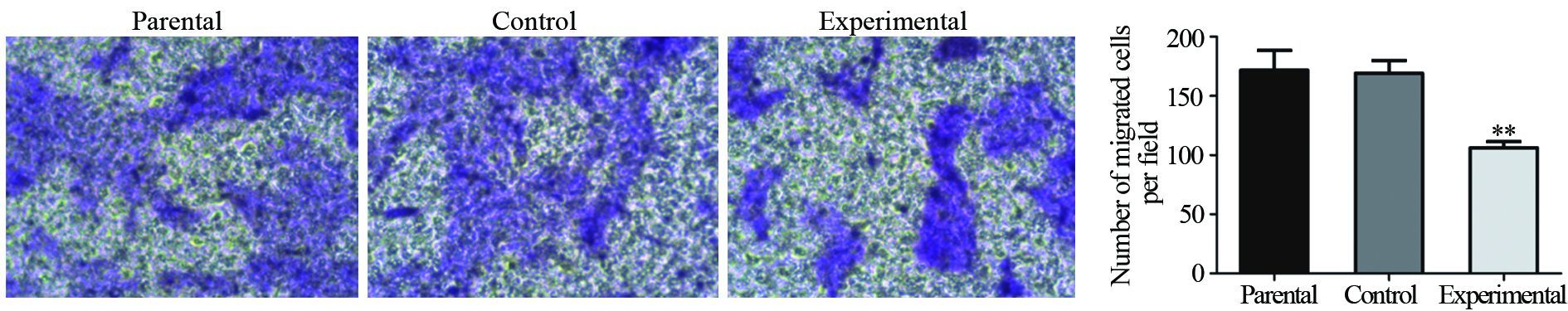
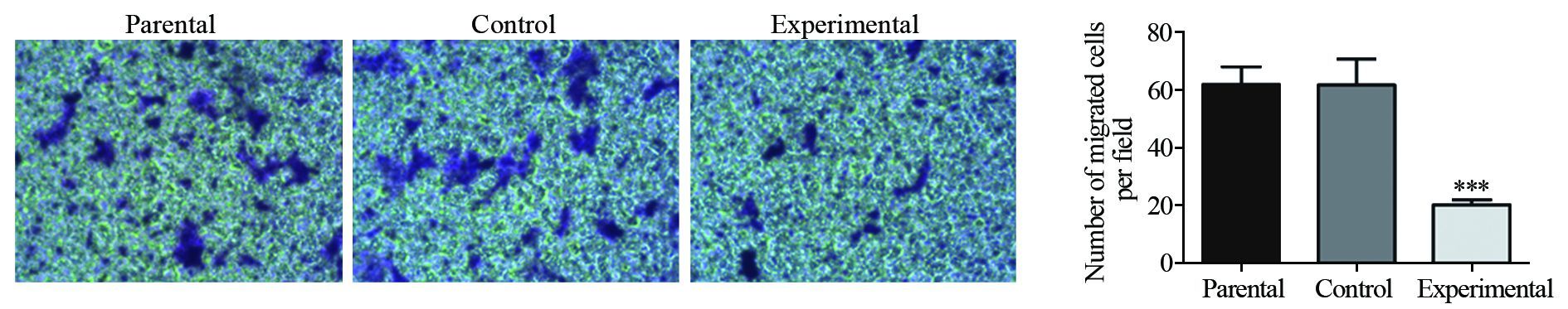
2.5 Transwell实验检测PAG1过表达对A431细胞运动能力的影响
Transwell迁移实验结果显示,亲本细胞组迁移的细胞数为(171.78±16.56)个,对照组为(169.3±10.62)个,实验组为(106.32±5.16)个,与划痕修复实验结果一致,实验组与对照组和亲本细胞组之间,差异有统计学意义(P=0.001),而亲本细胞组和对照组之间差异无统计学意义(P=0.964),见图 5。这表明,PAG1的过表达显著抑制了A431细胞的迁移能力。
Transwell侵袭实验结果,侵袭的细胞数分别为亲本细胞组(61.80±6.13)、对照组(61.54±9.05)、实验组(20.87±1.71),各组Transwell侵袭细胞数目均少于其Transwell迁移细胞数目,但结果与Transwell迁移实验一致,实验组与对照组和亲本细胞组之间,差异有统计学意义(P=0.000),亲本细胞组和对照组之间差异无统计学意义(P=0.962),见图 6。
3 讨论
高危型皮肤鳞状细胞癌是一类具有严重转移倾向的皮肤鳞状细胞癌,可以导致严重的致残率和致死率[3],其转移率甚至可以与肾癌、口咽癌及黑色素瘤转移率相匹[3]。大量研究报告中,临床医生和研究者们试图总结高危型cSCC的特征及其预后,但是关于高危型cSCC如何定义现仍没有达成共识。美国的全国癌症综合网(NCCN)和美国癌症联合委员会(AJCC)对于判断病损是否为高危型的标准并不一致,而且也没有可靠数据支持自己的定义比另一方更标准。鉴于cSCC生物学行为的差异性,若早期能够识别高危型cSCC,明确其临床分期,对于防止其复发和转移意义重大。
随着分子遗传学及细胞生物学的发展,人们对肿瘤形成过程进行了大量的研究,发现肿瘤形成受许多调节因子控制,如生长因子、信号转导蛋白和转录因子、细胞或基质间黏附因子。越来越多研究证据表明跨膜接头蛋白在生理和病理条件下发挥着重要作用,如免疫性疾病和肿瘤转化过程[4-5]。PAG1是2000年由两个独立实验小组几乎同时报道的一种脂筏相关的跨膜接头蛋白[6, 7],肿瘤相关性是其研究的热点。目前研究认为,PAG1通过调节Src家族成员激酶活性参与肿瘤的病理过程,其调节机制根据肿瘤细胞类型而异[2]。其中有一条机制认为Src被激活,活化的Src磷酸化PAG1的Y314位点,招募Csk接近Src,从而使Csk磷酸化Src羧基端527位的酪氨酸,使得Src本身的SH2结构域与磷酸化的位点结合,致使Src成为一种“关闭”状态,失去活性[8]。
本研究为了探讨PAG1对皮肤鳞癌的作用,通过转染将PAG1基因导入皮肤鳞癌细胞A431细胞,FCM检测转染率,并采用实时荧光定量PCR法进一步验证外源PAG1基因成功整合到A431细胞转录组,观察PAG1对A431细胞运动能力的影响。划痕修复实验结果显示PAG1过表达明显抑制A431细胞愈合率,同时在Transwell迁移实验中实验组中细胞迁移数目显著低于亲本细胞组和对照组,均表明外源性PAG1抑制了A431细胞迁移能力,Transwell侵袭实验结果显示PAG1过表达可降低A431细胞的侵袭能力。这提示A431细胞中,上调的PAG1能降低肿瘤的侵袭和转移能力。Kanou研究表明,在c-Src上调的非小细胞肺癌细胞系中,上调PAG1表达能抑制非小细胞肺癌体外侵袭及体内转移能力[9]。也有报道在结肠癌和食管癌组织中PAG1表达下调,导入外源性PAG1基因后,可以影响肿瘤的侵袭特性[10-11]。这些结论与本实验结果可相互佐证。推测PAG1主要由SFK(Src family kinase)磷酸化激活后招募Csk到脂筏上,进而通过调控SFKs(Src family kinases)活性阻断下游信号转导,参与细胞转化和恶性疾病发展。
综上所述,PAG1基因过表达可能通过改变人皮肤鳞状细胞癌的运动能力而抑制肿瘤浸润及远处转移,其相关机制及调控环节本课题组正在深入探讨中。在肿瘤的发生发展过程中,能发挥高效抑制作用的基因及蛋白是国内外研究者们一直追寻和探索的方向。希望围绕PAG1基因及蛋白表达功能的研究能够为未来高危型皮肤鳞状细胞癌的诊断、明确临床分期及治疗提供实验和理论依据。
-
-
[1] Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012[J]. J Am Acad Dermatol, 2013, 68(6): 957-66. doi: 10.1016/j.jaad.2012.11.037
[1] Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012[J]. J Am Acad Dermatol, 2013, 68(6): 957-66. [2] Hrdinka M, Horejsi V. PAG--a multipurpose transmembrane adaptor protein[J]. Oncogene, 2014, 33(41): 4881-92. doi: 10.1038/onc.2013.485
[2] Hrdinka M, Horejsi V. PAG--a multipurpose transmembrane adaptor protein[J]. Oncogene, 2014, 33(41): 4881-92. [3] Parikh SA, Patel VA, Ratner D. Advances in the management of cutaneous squamous cell carcinoma[J]. F1000Prime Rep, 2014, 6: 70. http://cn.bing.com/academic/profile?id=2145298090&encoded=0&v=paper_preview&mkt=zh-cn
[3] Parikh SA, Patel VA, Ratner D. Advances in the management of cutaneous squamous cell carcinoma[J]. F1000Prime Rep, 2014, 6: 70. [4] Svec A. Expression of transmembrane adaptor protein PAG/Cbp in diffuse large B-cell lymphoma: immunohistochemical study of 73 cases[J]. Pathol Res Pract, 2007, 203(4): 193-8. [4] Svec A. Expression of transmembrane adaptor protein PAG/Cbp in diffuse large B-cell lymphoma: immunohistochemical study of 73 cases[J]. Pathol Res Pract, 2007, 203(4): 193-8. doi: 10.1016/j.prp.2007.01.005
[5] Tauzin S, Ding H, Khatib K, et al. Oncogenic association of the Cbp/PAG adaptor protein with the Lyn tyrosine kinase in human B-NHL rafts[J]. Blood, 2008, 111(4): 2310-20. doi: 10.1182/blood-2007-05-090985
[5] Tauzin S, Ding H, Khatib K, et al. Oncogenic association of the Cbp/PAG adaptor protein with the Lyn tyrosine kinase in human B-NHL rafts[J]. Blood, 2008, 111(4): 2310-20. [6] Brdicka T, Pavlistová D, Leo A, et al. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation[J]. J Exp Med, 2000, 191(9): 1591-604. doi: 10.1084/jem.191.9.1591
[6] Brdicka T, Pavlistová D, Leo A, et al. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation[J]. J Exp Med, 2000, 191(9): 1591-604. [7] Kawabuchi M, Satomi Y, Takao T, et al. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases[J]. Nature, 2000, 404(6781): 999-1003. doi: 10.1038/35010121
[7] Kawabuchi M, Satomi Y, Takao T, et al. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases[J]. Nature, 2000, 404(6781): 999-1003. [8] Lindquist JA, Simeoni L, Schraven B. Transmembrane adapters: attractants for cytoplasmic effectors[J]. Immunol Rev, 2003, 191: 16 5-82. [8] Lindquist JA, Simeoni L, Schraven B. Transmembrane adapters: attractants for cytoplasmic effectors[J]. Immunol Rev, 2003, 191: 165-82. doi: 10.1034/j.1600-065X.2003.00007.x
[9] Kanou T, Oneyama C, Kawahara K, et al. The transmembrane adaptor Cbp/PAG1 controls the malignant potential of human non-small cell lung cancers that have c-src upregulation[J]. Mol Cancer Res, 2011, 9(1): 103-14. doi: 10.1158/1541-7786.MCR-10-0340
[9] Kanou T, Oneyama C, Kawahara K, et al. The transmembrane adaptor Cbp/PAG1 controls the malignant potential of human non-small cell lung cancers that have c-src upregulation[J]. Mol Cancer Res, 2011, 9(1): 103-14. [10] Zhou D, Dong P, Li YM, et al. Overexpression of Csk-binding protein decreases growth, invasion, and migration of esophageal carcinoma cells by controlling Src activation[J]. World J Gastroenterol, 2015, 21(6): 1814-20. [10] Zhou D, Dong P, Li YM, et al. Overexpression of Csk-binding protein decreases growth, invasion, and migration of esophageal carcinoma cells by controlling Src activation[J]. World J Gastroenterol, 2015, 21(6): 1814-20. doi: 10.3748/wjg.v21.i6.1814
[11] Oneyama C, Hikita T, Enya K, et al. The lipid raft-anchored adaptor protein Cbp controls the oncogenic potential of c-Src[J]. Mol Cell, 2008, 30(4): 426-36. [11] Oneyama C, Hikita T, Enya K, et al. The lipid raft-anchored adaptor protein Cbp controls the oncogenic potential of c-Src[J]. Mol Cell, 2008, 30(4): 426-36. doi: 10.1016/j.molcel.2008.03.026



 下载:
下载: