Quality Assessment of Reports of Randomized Controlled Trials Published in Chinese in Field of Non-small Cell Lung Cancer in China
-
摘要:目的
采用CONSORT声明对非小细胞肺癌(NSCLC)领域发表的中文随机对照试验(RCT)报告质量进行客观评估,分析其报告情况及存在的问题。
方法计算机检索中国生物医学文献数据库(CBM, 1978—2013.6)、中国知网(CNKI, 1979—2013.6)、维普数据库(VIP, 1989—2013.6)、万方数据库( WF, 1982—2013.6),纳入中文发表的NSCLC的RCT,采用CONSORT清单(2010版)进行质量评价,并分析纳入RCT报告质量的影响因素,所有数据录入Excel 2007软件,采用SPSS19.0软件进行统计分析。
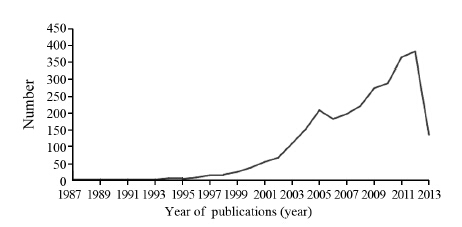
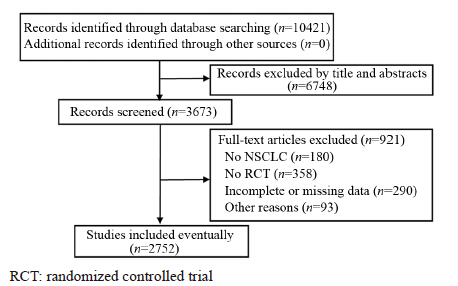
结果检索获得10 421条记录,最终纳入2 752篇RCT,评价结果显示NSCLC RCT报告质量存在缺陷,40.54%的条目完整报告率在30%及以下。分层分析结果显示,发表年代(2010年之前 vs. 2010年及之后)、单位数量(1个 vs. ≥2个)、杂志级别(CSCD vs. 非CSCD)、论文页数(<中位数 vs. ≥中位数)和基金资助(有基金 vs. 无基金)5个分层因素对50%及以上的CONSORT条目的报告有影响(P<0.05),其中以论文页数对条目报告的影响最大。
结论目前发表的中文NSCLC RCT整体报告质量不高,主要体现在对于方法学的描述和对结果的分析呈现都很不规范。作者单位数、CONSORT(2010版)的发布、论文页数、资金支持等均对文献报告质量有影响,在以后的研究中应该遵循CONSORT规范,提升NSCLC RCT的报告质量。
Abstract:ObjectiveTo explore the reporting quality and potential problems of randomized controlledtrials(RCT) published in Chinese in the field of non-small cell lung cancer(NSCLC) by CONSORTStatement.
MethodsCBM, CNKI, VIP, Wanfang database were systematically searched to identify RCTsof NSCLC. The deadline of the searching was June 2013. Date extraction table was designed according toCONSORT-2010 Statement, and the data were input into Excel 2007. Statistical analysis was performedusing SPSS19.0.
ResultsA total of 10421 references were identified, and 2752 RCTs were includedaccording to the inclusion and exclusion criteria. The assessment results showed that the reporting qualityof included studies had some problems. 40.54% items had a full report rate at ≤30%. Stratified analysisshowed that more than 50% items of CONSORT were associated with the year of publication (<2010 vs. ≥2010), the number of affiliations (1 vs. ≥2), journals indexed (CSCD vs. non CSCD), the median numberof paper page (<2 vs. ≥2), and fund (fund vs. non-funded) (P<0.05), moreover, the effect of paper page wasthe most obvious(P<0.00001).
ConclusionThe NSCLC RCT published in Chinese academic journals areinadequately reported, especially in the description of methods and presentation of results. The number ofunits, CONSORT (2010) published, the paper pages and financial support affect the quality of the reports. TheCONSORT statement should be complied in future studies to enhance the quality of NSCLC RCT.
-
Key words:
- CONSORT /
- Randomized controlled trials /
- Non-small cell lung cancer /
- Reporting quality
-
-
表 1 纳入2752篇RCT的CONSORT评价结果及不同分层因素报告质量比较
Table 1 Comparison of CONSORT evaluation results and different factors of report quality in 2752 randomized controlled trial(RCTs)

-
[1] Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015[J]. CA Cancer J Clin, 2015, 65(1): 5-29. [1] Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015[J]. CACancer J Clin, 2015, 65(1): 5-29.
[2] Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008[J]. Int J Cancer, 2010, 12 7(12): 2893-917. [2] Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burdenof cancer in 2008: GLOBOCAN 2008[J]. Int J Cancer, 2010,127(12): 2893-917.
[3] Zhou C. Lung cancer molecular epidemiology in China: recent trends[J]. Transl Lung Cancer Res, 2014, 3(5): 270-9. [3] Zhou C. Lung cancer molecular epidemiology in China: recenttrends[J]. Transl Lung Cancer Res, 2014, 3(5): 270-9.
[4] 侯恩存, 陈杰,甘露, 等. 康莱特联合GP方案治疗晚期非小细胞肺癌疗效的系统评价[J]. 现代肿瘤医学, 2015, 23(7): 960-5. Hou EC, Chen J, Gan L, et al. A systematic review of Kanglaite plusGP regimen in treating advanced non-small cell lung cancer[J].Xian Dai Zhong Liu Yi Xue, 2015, 23(7): 960-5.
[4] Hou EC, Chen J, Gan L, et al. A systematic review of Kanglaite plus GP regimen in treating advanced non-small cell lung cancer[J]. Xian Dai Zhong Liu Yi Xue, 2015, 23(7): 960-5. [侯恩存, 陈杰, 甘露, 等. 康莱特联合GP方案治疗晚期非小细胞肺癌疗效的系 统评价[J]. 现代肿瘤医学, 2015, 23(7): 960-5.] [5] 金玲, 徐萌, 刘畅, 等. 中医益气养阴解毒复方联合化疗治疗非小细胞肺癌的系统评价[J]. 中国老年学杂志,2015, 35(7): 1837-40. Jin L, Xu M, Liu C, et al. Effectiveness and safety of Yiqi YangyinJiedu recipe combined with chemotherapy for non-small cell lungcancer:a Meta-analysis[J]. Zhongguo Lao Nian Xue Za Zhi, 2015,35(7): 1837-40.
[5] Jin L, Xu M, Liu C, et al. Effectiveness and safety of Yiqi Yangyin Jiedu recipe combined with chemotherapy for non-small cell lung cancer:a Meta-analysis[J]. Zhongguo Lao Nian Xue Za Zhi, 2015, 35 (7): 1837-40. [金玲, 徐萌, 刘畅, 等. 中医益气养阴解毒复方 联合化疗治疗非小细胞肺癌的系统评价[J]. 中国老年学杂志, 20 15, 35(7): 1837-40.] [6] Altman DG, Schulz KF, Moher D, et al. The Revised CONSORT Statement for Reporting Randomized Trials: Explanation and Ela boration (1)[J]. Zhongguo Xun Zheng Yi Xue Za Zhi, 2005, 5(9): 71 2-4. [Altman DG, Schulz KF, Moher D, 等. 报告随机对照试 验的CONSORT声明修订版:说明与详述(一)[J]. 中国循证医学 杂志, 2005, 5(9): 712-4.] [6] Altman DG, Schulz KF, Moher D, 等. 报告随机对照试验的CONSORT声明修订版:说明与详述(一)[J]. 中国循证医学杂志, 2005, 5(9): 712-4.] Altman DG, Schulz KF, Moher D, et al. The Revised CONSORTStatement for Reporting Randomized Trials: Explanation and Elaboration (1)[J]. Zhongguo Xun Zheng Yi Xue Za Zhi, 2005, 5(9):712-4.
[7] 徐航, 秦纹,甘为, 等. 应用CONSORT声明对随机对照试验报告的质量分析[J]. 中国全科医学, 2012, 15(20): 2304-10. Xu H, Qin W, Gan W, et al. Analysis of the Quality of Reports ofRandomized Controlled Trials Using CONSORT Statement [J].Zhongguo Quan Ke Yi Xue, 2012, 15(20): 2304-10.
[7] Xu H, Qin W, Gan W, et al. Analysis of the Quality of Reports of Randomized Controlled Trials Using CONSORT Statement [J]. Zhongguo Quan Ke Yi Xue, 2012, 15(20): 2304-10. [徐航, 秦纹, 甘为, 等. 应用CONSORT声明对随机对照试验报告的质量分 析[J] 中国全科医学, 2012, 15(20): 2304-10.] [8] 董稳航, 李春洁, 项陈洋, 等. 我国口腔颌面外科临床随机对照试验的报告质量评价[J]. 华西口腔医学杂志, 2012, 30(5): 505-8. Dong WH, Li CJ, Xiang CY, et al. Quality assessment on reports of randomized controlled trials of oral and maxillofacial surgery in China[J]. Hua Xi Kou Qiang Yi Xue Za Zhi, 2012, 30(5): 505-8.
[8] Dong WH, Li CJ, Xiang CY, et al. Quality assessment on reports of randomized controlled trials of oral and maxillofacial surgery in China[J]. Hua Xi Kou Qiang Yi Xue Za Zhi, 2012, 30(5): 505-8. [董 稳航, 李春洁, 项陈洋, 等. 我国口腔颌面外科临床随机对照试 验的报告质量评价[J]. 华西口腔医学杂志, 2012, 30(5): 505-8.] [9] Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration:updated guidelines for reporting parallel group randomised trials[J]. BMJ, 2010, 340: c869.
[9] Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration:updated guidelines for reporting parallel group randomised trials[J]. BMJ, 2010, 340: c869. [10] Ren F, Xu HQ. Quality Evaluation of the Report for Randomized Controlled Trials of HuangQi Injection in Treating Diabetic Nephropathy[J]. Xi Bu Zhong Yi Yao, 2013, 26(12): 1-4. [任芳, 徐厚谦. 黄芪注射液治疗糖尿病肾病随机对照试验报告质量评 价[J] 西部中医药, 2013, 26(12): 1-4.] [10] 任芳,徐厚谦. 黄芪注射液治疗糖尿病肾病随机对照试验报告质量评价[J]. 西部中医药, 2013, 26(12): 1-4. Ren F, Xu HQ. Quality Evaluation of the Report for RandomizedControlled Trials of HuangQi Injection in Treating DiabeticNephropathy[J]. Xi Bu Zhong Yi Yao, 2013, 26(12): 1-4.
[11] Jemal A , Bray F, Center MM, et al. Global cancer statistics[J]. CA Cancer J Clin, 2011, 61(2): 69-90. [11] Jemal A , Bray F, Center MM, et al. Global cancer statistics[J]. CA Cancer J Clin, 2011, 61(2): 69-90.
[12] Liu HL, Zheng J, Li Z, et al. Quality assessment of the reports of randomized controlled trials of laparoscopic gastrectomy for gastric cancer published in China[J]. Zhonghua Qiang Jing Wai Ke Za Zhi(Dian Zi Ban), 2014, 7(2): 100-4. [刘海龙, 郑洁, 李桢 等. 国内期刊腹腔镜胃癌手术随机对照试验报告质量评价[J]. 中华腔镜外科杂志(电子版), 2014, 7(2): 100-4.] [12] 刘海龙, 郑洁, 李桢 等. 国内期刊腹腔镜胃癌手术随机对照试验报告质量评价[J].中华腔镜外科杂志(电子版), 2014, 7(2): 100-4. Liu HL, Zheng J, Li Z, et al. Quality assessment of the reportsof randomized controlled trials of laparoscopic gastrectomy forgastric cancer published in China[J]. Zhonghua Qiang Jing WaiKe Za Zhi(Dian Zi Ban), 2014, 7(2): 100-4.
[13] Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of biasdimensions of methodological quality associated with estimates of treatment effects in controlled trials[J]. JAMA, 1995,273(5): 408-12.
[13] Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of biasdimensions of methodological quality associated with estimates of treatment effects in controlled trials[J]. JAMA, 1995, 27 3(5): 408-12. [14] Altman DG, Schulz KF, Moher D, 等. 报告随机对照试验的CONSORT声明修订版:说明与详述(四)[J]. 中国循证医学杂志, 2005, 5(12): 941-3. Altman DG, Schulz KF, Moher D, et al. The Revised CONSORT Statement for Reporting Randomized Trials: Explanation and Ela boration (4)[J]. Zhongguo Xun Zheng Yi Xue Za Zhi, 2005, 5(12):941-3.
[14] Altman DG, Schulz KF, Moher D, et al. The Revised CONSORT Statement for Reporting Randomized Trials: Explanation and Ela boration (4)[J]. Zhongguo Xun Zheng Yi Xue Za Zhi, 2005, 5(12): 94 1-3. [Altman DG, Schulz KF, Moher D, 等. 报告随机对照试 验的CONSORT声明修订版:说明与详述(四)[J]. 中国循证医 学杂志, 2005, 5(12): 941-3.] [15] Mills EJ, Wu P, Gagnier J, et al. The quality of randomized trialreporting in leading medical journals since the revised CONSORTstatement[J].Contemp Clin Trials, 2005, 26(4): 480-7.
[15] Mills EJ, Wu P, Gagnier J, et al. The quality of randomized trial reporting in leading medical journals since the revised CONSORT statement[J].Contemp Clin Trials, 2005, 26(4): 480-7. [16] Huwiler-Müntener K, Jüni P, Junker C, et al. Quality of reportingof randomized trials as a measure of methodologic quality[J].JAMA, 2002, 287(21): 2801-4.
[16] Huwiler-Müntener K, Jüni P, Junker C, et al. Quality of reporting of randomized trials as a measure of methodologic quality[J]. JAMA, 2002, 287(21): 2801-4. [17] Altman DG, Schulz KF, Moher D, et al. The Revised CONSORT Statement for Reporting Randomized Trials:Explanation and Ela boration(6)[J]. Zhongguo Xun Zheng Yi Xue Za Zhi, 2006, 6(2): 13 9-41. [Altman DG, Schulz KF, Moher D, 等. 报告随机对照试 验的CONSORT声明修订版:说明与详述(六)[J]. 中国循证医 学杂志, 2006, 6(2): 139-41.] [17] Altman DG, Schulz KF, Moher D, 等. 报告随机对照试验的CONSORT声明修订版:说明与详述(六)[J]. 中国循证医学杂志, 2006, 6(2): 139-41. Altman DG, Schulz KF, Moher D, et al. The Revised CONSORTStatement for Reporting Randomized Trials:Explanation and Elaboration(6)[J]. Zhongguo Xun Zheng Yi Xue Za Zhi, 2006, 6(2):139-41.
[18] Wu TX, Li Y, Bian Z, et al. Randomized trials published in some Chinese Journals: how many are randomized?[J]. Trials, 2009, 10 (1): 46-53. [18] Wu TX, Li Y, Bian Z, et al. Randomized trials published in someChinese Journals: how many are randomized?[J]. Trials, 2009,10(1): 46-53.
[19] Su M, Zhang LL, Li CB. Quality assessment of the reports of r andomized controlled trials published in 5 journals of psychia try in China from 2004 to 2008 by Consolidated Standards of Rep orting Trials Statement[J]. Zhonghua Jing Shen Ke Za Zhi, 2012, 45 (5): 263-7. [苏旻, 张兰兰, 李春波. 临床试验报告的统一标准 声明评价2004-2008年国内五种精神科期刊随机对照试验文献 报告质量[J]. 中华精神科杂志, 2012, 45(5): 263-7.] [19] 苏旻, 张兰兰, 李春波. 临床试验报告的统一标准声明评价2004-2008年国内五种精神科期刊随机对照试验文献报告质量[J]. 中华精神科杂志, 2012, 45(5): 263-7. Su M, Zhang LL, Li CB. Quality assessment of the reports of randomized controlled trials published in 5 journals of psychiatry in China from 2004 to 2008 by Consolidated Standards of Reporting Trials Statement[J]. Zhonghua Jing Shen Ke Za Zhi, 2012,45(5): 263-7.
[20] 马继春, 娜和亚, 毛婧, 等. 我国“循证"冠名期刊发表干预类系统评价/Meta分析的现状调查[J]. 中国循证医学杂志, 2013, 13(7): 896-900. Ma JC, Na HY, Mao J, et al. Status Survey on Systematic R e v i ew/Me t a - a n a l y s i s R e l a t e d t o I n t e r v e n t i o s n Chinese Journals Entitled with Evidence-Based[J]. Zhongguo Xun Zheng Yi Xue Za Zhi, 2013, 13(7): 896-900.
[20] Ma JC, Na HY, Mao J, et al. Status Survey on Systematic R e v i ew/Me t a - a n a l y s i s R e l a t e d t o I n t e r v e n t i o s n Chinese Journals Entitled with Evidence-Based[J]. Zhongguo Xun Zheng Yi Xue Za Zhi, 2013, 13(7): 896-900. [马继春, 娜和亚, 毛 婧, 等. 我国“循证”冠名期刊发表干预类系统评价/Meta分析 的现状调查[J]. 中国循证医学杂志, 2013, 13(7): 896-900.]



 下载:
下载: