Clinical Observation of Metronomic Oral Vinorelbine Monotherapy as First-line Treatment on Elderly Patients with Advanced Non-small Cell Lung Cancer
-
摘要:目的
探讨口服长春瑞滨软胶囊单药节拍化疗在老年非小细胞肺癌患者中的临床疗效及生存时间分析。
方法选择56例年龄≥75岁初治老年非小细胞肺癌患者, 给以口服长春瑞滨软胶囊50 mg每次, 每周3次单药节拍化疗, 观察临床疗效、不良反应及总生存期和无进展生存期。
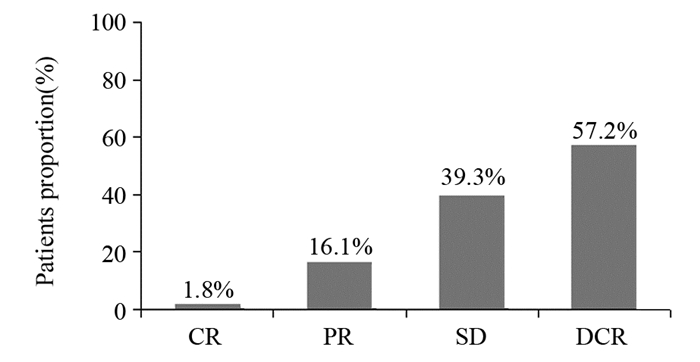
结果全部患者疗效评价CR 1例(1.8%), PR 9例(16.1%), ORR为17.9%;SD 22例(39.3%), 并且稳定时间人均达12周以上, DCR达57.2%;中位无进展生存期5月(3~22月), 中位总生存期9月(4~31月), 1年生存率为35.7%(20/56), 2年生存率为16.1%(9/56), 3~4级不良反应出现较少。
结论应用长春瑞滨软胶囊单药节拍化疗安全可靠, 可有效改善生活质量, 为老年非小细胞肺癌患者临床综合治疗决策提供了新的思路。
Abstract:ObjectiveTo investigate the clinical effect and survival analysis of metronomic oral vinorelbine monotherapy on elderly patients with advanced non-small cell lung cancer (NSCLC).
MethodsWe involved 56 chemotherapy-naive elderly patients(≥75 yrs) with stage ⅢB-Ⅳ NSCLC who received oral vinorelbine 50 mg per time, three times per week (Monday-Wednesday-Friday) continuously, until disease progression, unacceptable toxicity or patient refusal.Observation endpoints were overall response rate (ORR), overall survival(OS) and progression free survival(PFS).
ResultsORR was 17.9% with 9 cases(16.1%) of PR and 1 case(1.8%) of CR; 22 patients(39.3%) experienced stable disease lasting more than 12 weeks, leading to an overall DCR of 57.2%.Median progression-free survival was 5 months (range 3-22 months) and median overall survival was 9 months (range 4-31 months).Treatment was well tolerated with rare serious toxicity(grade 3-4).
ConclusionMetronomic oral vinorelbine monotherapy is safe, reliable and could improve patients' life of quality, which provide an interesting option for clinic therapy decision on elderly patients with advanced NSCLC.
-
Key words:
- Vinorelbine /
- Elderly /
- Non-small cell lung cancer /
- Metronomic chemotherapy
-
0 引言
胰腺癌虽然不是高发癌种,但却是第7大癌症导致死亡的原因。2020年,约有46.6万死亡病例,约占恶性肿瘤死亡4.7%。全球约47.1%的新发病例和48.1%的死亡病例发生在亚洲,其次是欧洲(新发病例占28.3%,死亡病例占28.4%)和北美洲(新发病例占12.6%,死亡病例占11.4%)[1]。各国胰腺癌的年龄标准化死亡率存在较大差异,欧盟、欧洲其他地区、北美、澳大利亚和新西兰、阿根廷、巴西和南非的死亡率最高。相反,非洲和南亚欠发达国家报告的死亡率很低,基本呈现发达国家胰腺癌的疾病负担高于发展中国家的差异[2]。这种地理和社会经济因素差异意味着环境因素和遗传因素在胰腺癌的发展中起着重要作用。
1 中国胰腺癌疾病负担
根据国家癌症中心报道的肿瘤登记数据,在我国最常被诊断的癌症中,胰腺癌位列第十,同时也是中国第六大癌症死亡原因。2015年共有9.5万胰腺癌新发病例和8.5万胰腺癌死亡病例,男性和城市地区的发病率和死亡率普遍较高[3]。根据2016年的肿瘤登记数据,胰腺癌的年龄别发病率和死亡率随着年龄增加而增加,44岁以前处于较低的水平,45~49岁阶段迅速上升,在80岁之后达到发病和死亡高峰。在地区分布上,胰腺癌的发病率和死亡率存在着东部地区高于中部和西部地区的差异[4]。
根据2009年—2019年《中国肿瘤登记年报》摘录2006年—2016年的胰腺癌发病和死亡中标率并绘制发病死亡趋势曲线可以看出,胰腺癌总体发病和死亡中标率均呈上升趋势。以2009年为界,胰腺癌的发病率与死亡率在2009年之前呈下降趋势,发病率由3.65/10万下降至3.35/10万,死亡率由3.35/10万下降至2.98/10万,2009年后发病率和死亡率开始上升,于2011年达到峰值(发病率:4.60/10万;死亡率:4.14/10万),见图 1~4。
2 中国胰腺癌基本临床诊疗特征
胰腺癌起病隐匿,早期症状不典型,常表现为消化不良、腹泻、上腹部不适或腰背部疼痛等症状,易与其他消化系统疾病混淆。中国胰腺疾病大数据中心数据显示,胰腺癌存在早期诊断率低、手术切除率低和药物有效率低的“三低”特征。61.7%的胰腺癌为胰头癌,其次是胰体尾癌占33.8%,胰颈癌占4.3%。80.1%的手术患者均采用了开腹手术[5]。
3 中国胰腺癌发生的危险因素
与子宫颈癌、肺癌等肿瘤不同,胰腺癌相关的危险因素多样而复杂。Huang等使用来自GLOBOCAN 2018数据库收录的184个国家胰腺癌发病率和死亡率数据,通过单变量和多变量线性回归分析了生活方式和代谢危险因素(从世界卫生组织全球健康观察数据库中提取)与胰腺癌发病率和死亡率之间的关系,确定了吸烟、饮酒、缺乏体育运动、肥胖等胰腺癌危险因素[6]。因此,我们就现有研究确定的危险因素对中国人群胰腺癌疾病负担的影响进行总结。
3.1 一般因素
胰腺癌是一种衰老性疾病,在老年人中发病率增加。研究表明,老年患者通常不太可能接受胰腺癌的治疗,并且预后结果更差,可能与老年相关疾病有关,如合并症、营养不良、身体和认知功能受损、社会支持有限等[7]。值得注意的是,我国胰腺癌的发病情况在近年来出现了年轻化的趋势,提示我们要重视年轻人群的发病机制和防控工作[8]。我国男性胰腺癌疾病负担高于女性,与全球的不同性别胰腺癌发病和死亡差异相同[1],且现有研究证据表明女性胰腺癌的发生与生殖因素关系不大,从侧面反映了男性可能受其他环境和行为危险因素影响较大,进而导致发病死亡率较女性高[9]。意外的是,大型流行病学研究发现ABO血型与胰腺癌发生风险之间存在联系。我国汉族人群的一项研究显示,A、AB或B血型的人群发生胰腺癌的风险比O血型的高,且O型血胰腺癌患者中位生存时间明显长于非O型血患者[10],反映了血型与胰腺癌发生与进展的相关性。
3.2 吸烟
国际癌症研究机构已确认吸烟与胰腺癌有因果关系[2],在吸烟强度、持续时间、吸烟累积量和胰腺癌风险之间也被证实存在非线性剂量-反应关系[11]。关于胰腺癌死亡率的相关性,一项研究对亚太地区30个队列研究(包括7项中国的队列研究)共计42万余名受试者的危险因素和胰腺癌死亡数据进行分析[12],在355.8万人年的随访中,吸烟被证实是胰腺癌死亡的独立危险因素,使胰腺癌死亡风险增加了10%(HR=1.10, 95%CI: 1.01~1.20)。中国Kadoorie生物样本库研究对51万受试者进行了9年的随访,证实了吸烟、饮酒是胰腺癌的危险因素,尤其是在男性和城市地区人群中,这种有害效应更加明显[13]。此外,我国上海一项在慢性胰腺炎患者中进行的随访研究表明,每年吸烟60包以上的患者胰腺癌发病风险更高,表明在我国胰腺癌高危人群中,戒烟有望降低胰腺癌的发病风险[14]。
3.3 饮酒
慢性酒精摄入可导致胰腺结构和功能损伤[15]。Wang等的Meta分析表明,低到中度的酒精摄入对胰腺癌的风险几乎没有影响,但高酒精摄入量与胰腺癌风险增加相关(RR=1.15, 95%CI: 1.06~1.25)[16]。一项中国人群饮酒与胰腺癌关系的Meta分析表明,饮酒行为与胰腺癌的发生具有显著的正相关性[17]。回顾我国的前瞻性研究,前文所述Kadoorie生物样本库研究证实了重度饮酒与男性胰腺癌发病的相关性[13];Zhang等的队列研究结果表明,曾经饮酒的胰腺癌患者死亡率较从未饮酒的胰腺癌患者死亡风险高25%[18]。中国现有关于饮酒与胰腺癌风险的研究报道不一,且当前研究大多为病例对照研究,因此,中国人群中饮酒与胰腺癌的关系还有待验证。
3.4 身体质量指数超标
世界癌症研究基金会(World Cancer Research Fund International, WCRF)与美国癌症研究所(American Institute of Cancer Research, AICR)工作组回顾了饮食、营养和体育锻炼对胰腺癌的影响,认为有“强有力的证据”表明超重、肥胖或身高过高会增加患胰腺癌的风险[19]。胰腺癌肿瘤微环境中的脂肪细胞在促炎过程中起着关键作用,通过与癌细胞和其他间质细胞的串扰机制促进胰腺癌的进展[20-21]。在中国进行的关于肥胖与胰腺癌关系的研究较少,一项研究对51 251例新加坡华人受试者进行前瞻性随访,结果发现,在曾经吸烟者中,身体质量指数(body mass index, BMI)低于18.5 kg/m2的人与BMI为21.5~24.4 kg/m2的吸烟者相比,患胰腺癌的风险增加了99%(HR=1.99, 95%CI: 1.03~-3.84)。从未吸烟者中,BMI≥27.5 kg/m2的受试者相对于BMI为21.5~24.4 kg/m2的受试者患胰腺癌的风险增加了75%(HR=1.75, 95%CI: 0.93~3.3)[22]。中国的一项队列研究调查了1 783例胰腺癌患者的BMI与胰腺癌生存率的关系,未发现两者有相关性[23]。
3.5 遗传因素及家族史
流行病学证据表明,胰腺癌家族史是胰腺癌的危险因素,且胰腺癌患者亲属的胰腺组织学常表现出多种癌前病变[24]。10%~20%的胰腺癌与遗传因素有关,某些致病基因的种系突变可发生遗传,导致家族聚集性胰腺癌[25-26]。家族聚集性胰腺癌的流行病学特征与散发胰腺癌具有一定差异,表现为有吸烟史或糖尿病史的家族性胰腺癌患者发病年龄显著提前[24]。中国胰腺癌综合诊治指南(2020版)建议,对存在一些胰腺癌易感基因ATM、BRCA1、BRCA2、CDKN2A、MLH1、MSH2、MSH6、EPCAM、PALB2、STK11、TP53等致病或可能致病的胚系突变,家族内具有胰腺癌病史(一级或二级亲属)的个体,推荐开展早期筛查[27]。此外,胰腺癌还与几种高度特征性遗传综合征相关,包括遗传性胰腺炎、家族性多发性非典型丘状黑色素瘤、Peutz-Jeghers综合征等[28]。这些综合征常伴有生殖细胞的某些基因突变。
3.6 饮食
饮食是一个重要的可改变的生活方式因素,但流行病学研究评估胰腺癌风险与个别营养素或食物(如红肉、加工肉类、蔬菜和水果以及相关的维生素和矿物质、纤维、脂肪和脂肪酸等)报告的结果不一致[29]。在中国,Pang等的前瞻性研究表明,每日食用新鲜水果可以使胰腺癌患病的风险降低34%,而每日食用红色肉类则使胰腺癌患病风险增加16%[13]。上海进行的一项以人群为基础的病例对照研究为中国目前规模较大的饮食与胰腺癌风险的研究,该研究纳入了908例胰腺癌患者和1 067例正常对照者,对其进行饮食方式相关分析显示,饮食的能量密度与患胰腺癌的风险正相关[30]。饮食能量密度是指饮食中单个元素的能量密度,如高脂肪食物为能量密度高的饮食,而水分含量高的食物构成能量稀释的饮食,例如新鲜水果和蔬菜、白肉、大豆和乳制品等。因此,该研究结论基本上与现有国际上的研究结论一致。
3.7 体力活动
体力活动可以对抗超重、肥胖和糖尿病等胰腺癌危险因素,还可能通过降低瘦素、C反应蛋白和白细胞介素-6等的水平,通过减少慢性炎性反应的机制预防胰腺癌[31]。我国关于体力活动与胰腺癌关系的研究较少。上海男性和女性健康研究队列发现,每周150分钟的中等强度运动或每周75分钟高强度运动使胰腺癌发病风险降低了41%,终身锻炼能够使胰腺癌风险降低68%,但未在女性中观察到体力活动与胰腺癌风险的相关性[32]。体力活动与胰腺癌风险的性别差异报道较少,根据现有流行病学证据,推测在女性中,雌激素增加和胰腺癌风险降低之间有潜在关联,而体育锻炼可能会降低女性雌激素水平[33],这可能是体力活动与女性胰腺癌风险无关的潜在原因。
3.8 其他疾病与感染因素
慢性胰腺炎是胰腺癌的重要危险因素。过往研究表明,慢性胰腺炎患者较未患慢性胰腺炎者胰腺癌发生率显著增加。患者首次确诊慢性胰腺炎的两年内,胰腺癌的发病风险增加16倍[34]。胰腺炎不仅可以通过激活炎性反应因子以辅助癌细胞逃避免疫系统的清除机制[35],还有相关报道表明慢性胰腺炎可以引发3c型糖尿病,进而提高胰腺癌患病风险[36]。国内外大量流行病学研究发现,糖尿病与胰腺癌风险增加呈正相关,30%~40%胰腺癌患者合并有糖尿病,80%有糖耐量异常[37]。另一项Meta分析纳入了中国的26项病例对照研究,共筛选出胰腺癌患者7 702例和对照人群10 186例。该研究显示,糖尿病患者患胰腺癌的风险是健康人群的3.69倍,并且胰腺癌发生的风险与糖尿病的持续时间具有相关性[38]。此外,现有研究表明诸如乙型肝炎病毒、丙型肝炎病毒、幽门螺杆菌等感染可通过影响胰腺功能增加胰腺癌发生风险[39],但国内尚缺乏大样本量前瞻性研究的报道,其关联性还有待验证。
4 中国胰腺癌的筛查与早期诊断
胰腺癌的发生时间较长,目前临床工作者致力于开发新的胰腺癌早期筛查和诊断方法研究,但绝大多数是在有病理证实的胰腺癌患者中,观察和评价不同诊断方法的敏感度、特异度和准确性,而真正意义上的早期诊断应提早到在细胞学和分子生物学的水平预测胰腺癌的发生之前,因此明确胰腺癌高危人群的定义并定期随访是提高早期胰腺癌诊断率的关键。胰腺癌为罕见恶性肿瘤,一般人群终身患病风险为1.3%,因此不建议把无症状人群设为筛查对象,选择胰腺癌的高危人群为筛查对象将获得更大的收益。国外将有胰腺癌家族史和(或)患有某些遗传综合征(如遗传性胰腺炎、遗传性非息肉病性结直肠癌、Peutz-Jeghers综合征、家族性乳腺癌、家族性非典型性多发性黑色素瘤等)的个体定义为高危人群进行筛查[40]。我国将胰腺癌的高危因素如不良生活方式(吸烟、肥胖、酗酒、三高饮食等)、良性疾病(慢性胰腺炎、糖尿病、消化道良性疾病手术史等)等非遗传因素,以及家族性胰腺癌、遗传性乳腺癌、遗传性胰腺炎、黑色素瘤综合征等遗传因素综合起来制定了胰腺癌高危人群的筛查量表,以便针对不同患病风险人群制定筛查策略[41]。
目前国内对高危人群的鉴定和筛查尚无公认的方案,虽然胰腺癌筛查手段在不断发展,但从卫生经济学的角度来讲,现有筛查方式对疾病负担和诊疗成本的降低效果并不明显。目前,胰腺癌的筛查手段主要为影像学、肿瘤标志物和基因检测。超声、CT、MRI、MRCP、EUS等传统影像学技术在不断更迭。超声因其经济、简单、无创等特点,是早年间胰腺癌高危人群筛查的首选影像学手段,随后,CT检查逐渐成为广泛应用于胰腺癌诊断、分期、治疗效果评价中使用最多的手段[42]。然而,虽然CT、MRI等影像学检查具有重要价值,但是因价格昂贵、操作复杂,因此难以在高危人群中进行广泛应用。2020年我国最新综合诊疗指南推荐高危人群每年进行一次增强CT、MRI、磁共振胰胆管造影(MRCP)和(或)超声内镜(EUS)检查,可以缩短可疑个体筛查时间[27]。然而,该筛查手段的成本较高,医保所能报销的范围较小,需证据等级高的卫生经济学研究来进行成本-效益分析。另外,由于胰腺癌病理分型不同,不同影像学技术的敏感度和特异度表现出较大差异,难以选择出最合适的影像学技术应用于实际筛查工作中,这也是限制影像学作为胰腺癌主要筛查手段的重要因素。荷兰家族聚集性胰腺癌监测研究的结果表明,EUS对胰腺实体病灶检查敏感度远远优于MRI/MRCP,但在囊性病变中,后者优于前者[43]。
胰腺癌的肿瘤标志物方面,单一肿瘤标志物诊断胰腺癌,尤其是诊断早期胰腺癌的敏感度和特异度均不高[44]。CA19-9是胰腺癌的相关抗原,对晚期胰腺癌的诊断具有重要价值,但早期胰腺癌患者血清中CA19-9的水平不高,且CA19-9在某些良性病变或其他恶性肿瘤中也可能发生高表达,因此CA19-9必须与其他肿瘤标志物联合应用[45-46]。目前,国内的一项Meta分析表明,CA242对胰腺癌诊断的敏感度(71.9%)和特异度(86.8%)更高[47]。相关组学研究也提及了一些潜在的胰腺癌早期诊断标志物,使用代谢组学方法研究发现,在胰腺癌疾病进程中,发生了代谢重编程、对微环境中其他代谢物质进行代谢干扰等生物过程,因此多种代谢产物如M2-丙酮酸激酶、异柠檬酸、肌醇有望成为胰腺癌早期诊断的标志物[48-49]。目前,针对胰腺癌肿瘤标志物的研究较多,如若将多种肿瘤标志物联合检测,可弥补单一指标的局限性并互补各指标的优缺点。因此,建立胰腺癌早期诊断的多种肿瘤标志物预测模型,是提高诊断效能的潜在方法。
目前较为成熟的胰腺癌基因检测为针对K-ras基因、p53基因突变的检测。K-ras基因的突变与胰腺癌的发生最为密切,且该基因的突变主要发生在早期阶段,因此可以作为早期诊断的重要手段,但其诊断效能不尽如人意[50]。而p53基因突变的检测对高危人群筛选的潜在价值已经得到证实,目前主要针对其检测方法进行改进,以提高诊断价值[51]。关于微小RNA、ctDNA、外泌体等与胰腺癌诊断的研究为胰腺癌的发病机制以及早期诊断提供了线索,但其在临床和公共卫生领域的应用价值仍需要大样本量的前瞻性研究进行验证。
5 总结
目前对于胰腺癌的病因、自然进程以及预防措施仍知之甚少。吸烟是多种肿瘤的危险因素,也是目前唯一确定的胰腺癌独立危险因素。尚无高等级的研究证据验证诸如超重/肥胖、有家族史、糖尿病等因素,因此,现有危险因素研究暂不足以进行胰腺癌高危人群的精准划分。而高危人群的筛查可以有效降低筛查成本和提高检出效率,更适合我国国情。因此,建立适合我国人群的胰腺癌高危人群评估系统和在公共卫生资源不足条件下最具卫生经济效益的筛查方式是我国未来胰腺癌防控的发展方向。
-
表 1 入组患者基线临床资料(n=56)
Table 1 Baseline clinical characteristics of elderly patients with advanced non-small cell lung cancer (NSCLC) (n=56)

表 2 长春瑞滨软胶囊单药节拍化疗不良反应(n(%))
Table 2 Treatment-related toxicities at final analysis of metronomic oral vinorelbine monotherapy (n(%))

-
[1] 陈万青, 李贺, 孙可欣, 等. 2014年中国恶性肿瘤发病和死亡分析[J].中华肿瘤杂志, 2018, 40(1):5-13. http://d.old.wanfangdata.com.cn/Periodical/zhzl201801002 Chen WQ, Li H, Sun KX, et al.Report of Cancer Incidence and Mortality in China, 2014[J].Zhonghua Zhong Liu Za Zhi, 2018, 40(1):5-13. http://d.old.wanfangdata.com.cn/Periodical/zhzl201801002
[2] Besse B, Adjei A, Baas P, et al.2nd ESMO Consensus Conference on Lung Cancer:non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease[J].Ann Oncol, 2014, 25(8):1475-84. doi: 10.1093/annonc/mdu123
[3] Maher J.Silver survivors:how do we know if people are 'too old' for cancer treatment?[J].Future Oncol, 2014, 10(11):1811-3. doi: 10.2217/fon.14.118
[4] GBD 2013 Mortality and Causes of Death Collaborators.Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013:a systematic analysis for the Global Burden of Disease Study 2013[J].Lancet, 2015, 385(9963):117-71. doi: 10.1016/S0140-6736(14)61682-2
[5] Santos FN, de Castria TB, Cruz MR, et al.Chemotherapy for advanced non-small cell lung cancer in the elderly population[J].Cochrane Database Syst Rev, 2015, (10):CD010463. http://europepmc.org/abstract/MED/26482542
[6] Kerbel RS, Kamen BA.The anti-angiogenic basis of metronomic chemotherapy[J].Nat Rev Cancer, 2004, 4(6):423-36. doi: 10.1038/nrc1369
[7] Gnoni A, Silvestris N, Licchetta A, et al.Metronomic chemotherapy from rationale to clinical studies:a dream or reality?[J].Crit Rev Oncol Hematol, 2015, 95(1):46-61. doi: 10.1016/j.critrevonc.2015.01.008
[8] D'Addario G, Früh M, Reck M et al.Metastatic non-small-cell lung cancer:ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up[J].Ann Oncol, 2010, Suppl 5:v116-9. http://europepmc.org/abstract/MED/20555059
[9] Bennouna J, Havel L, Krzakowski M, et al.Oral vinorelbine plus cisplatin as first-line chemotherapy in nonsquamous non-small-celllungcancer:final results of an International randomized phase Ⅱ study (NAVotrial 01)[J].Clin Lung Cancer, 2014, 15(4):258-65. doi: 10.1016/j.cllc.2014.04.007
[10] Martoni A, Marino A, Sperandi F, et al.Multicentre randomised phase Ⅲ study comparing the same dose and schedule of cisplatin plus the same schedule of vinorelbine or gemcitabine in advanced non-small cell lung cancer[J].Eur J Cancer, 2005, 41(1):81-92. doi: 10.1016/j.ejca.2004.08.029
[11] Tan EH, Rolski J, Grodzki T, et al.Global Lung Oncology Branch trial 3 (GLOB3):final results of a randomised multinational phase Ⅲ study alternating oral and i.v.vinorelbine plus cisplatin versus docetaxel plus cisplatin as first-line treatment of advanced non-small-cell lung cancer[J].Ann Oncol, 2009, 20(7):1249-56. doi: 10.1093/annonc/mdn774
-
期刊类型引用(46)
1. 蓝桂莲,吴遥,祝荫. 1990-2021年中国消化道肿瘤发病率与死亡率的趋势及未来20年预测分析. 中华肿瘤防治杂志. 2025(02): 84-92 .  百度学术
百度学术
2. 石智尧,武时瑜,任少键,刘懿禅,尹艺杰,高宇,王晞星. 胰腺癌中医证候分布规律及影响因素分析. 临床肝胆病杂志. 2025(03): 528-535 .  百度学术
百度学术
3. 卓宇果,高青. 经病理证实的163例胰头占位性病变患者的临床分析. 胃肠病学和肝病学杂志. 2024(01): 73-77 .  百度学术
百度学术
4. 张芝,王伟. 增强CT对胰腺癌术前诊断的价值与影像特征分析. 影像研究与医学应用. 2024(02): 136-138 .  百度学术
百度学术
5. 王书航,吴晓阳. 内质网蛋白驻留受体2蛋白对胰腺癌恶性生长的影响及机制研究. 江苏大学学报(医学版). 2024(02): 118-131 .  百度学术
百度学术
6. 李越,吕红艳,梁天宇,王郅宜,张若宣,贾智,李泉旺. 基于络病理论探讨胰腺癌炎癌转化的防治. 现代中医临床. 2024(02): 69-72 .  百度学术
百度学术
7. 吕尚泽,王贵明. 老年胰腺癌临床病理特征及预后相关因素分析. 临床消化病杂志. 2024(02): 77-81 .  百度学术
百度学术
8. 马红,闫弘颖,杨晓杰,祁颖. 温脾化瘀汤联合化疗对胰腺癌患者肿瘤标志物水平及预后的影响. 大医生. 2024(05): 20-22 .  百度学术
百度学术
9. 李敏红,李志铭,陈淮,余林,梁杰锋,列潮炜. 基于深度学习算法的胰腺癌CT自动分期系统的构建与应用. 现代肿瘤医学. 2024(11): 2055-2059 .  百度学术
百度学术
10. 曹阳,李前,崔文慧,王亚玲,司鑫鑫. 新型1H-吡咯[2, 3-c]并吡啶衍生物的合成和抗胰腺癌活性评价. 精细化工中间体. 2024(03): 31-37 .  百度学术
百度学术
11. 伍嘉颖,张彦军. 肠道菌群与胰腺癌发病机制及诊治的研究进展. 西部医学. 2024(07): 1088-1092 .  百度学术
百度学术
12. 董源涛,尚金红,袁燕丽. MRI联合CT在胰腺肿瘤中的诊断价值. 影像研究与医学应用. 2024(16): 137-139 .  百度学术
百度学术
13. 金禹辰,刘馨怡,岑章敏,谌泽芳,张振,刘珍,邓天好. 基于数据挖掘探析国医大师潘敏求治疗胰腺癌的用药规律及学术思想. 中医药导报. 2024(07): 129-134 .  百度学术
百度学术
14. 汪根良,范红娟,李健. AFF3在胰腺癌组织中的表达及与免疫浸润和患者预后的相关性. 国际消化病杂志. 2024(04): 274-278 .  百度学术
百度学术
15. 李俊,王震,李翠娟,巩振东,呼睿,郑容镡,冯盟盟. 唾液检测在中西医病证诊疗中的应用研究进展. 现代中医药. 2024(06): 1-6 .  百度学术
百度学术
16. 张韵致,陈琳琳. 青年胰腺癌患者疾病特异生存列线图的构建. 医药前沿. 2024(04): 1-4+8 .  百度学术
百度学术
17. 王雅,卢义晨. 白蛋白紫杉醇联合吉西他滨与替吉奥联合吉西他滨治疗转移性胰腺癌的疗效及安全性对比. 生命科学仪器. 2024(06): 97-98+101 .  百度学术
百度学术
18. 杨梦玲,黄辉,程烨. 黄芪多糖联合放化疗治疗多类肿瘤的Meta分析. 中西医结合心血管病电子杂志. 2024(03): 115-122+67 .  百度学术
百度学术
19. 朱燃培,张亚玲,魏丹丹,郑玉玲. 郑玉玲治疗胰腺癌的用药规律及学术思想分析. 中医学报. 2023(02): 327-335 .  百度学术
百度学术
20. 李威倩,陈奕明,张文婷,苏莹珍,帅红艳,Yu Xin. 基于生物信息学分析筛选胰腺癌差异表达基因. 大理大学学报. 2023(02): 24-30 .  百度学术
百度学术
21. 黄坤,何运胜,李建波,赵攀,肖春波,赵平武. 胰腺癌肝转移核心基因的筛选与验证. 中国普通外科杂志. 2023(03): 390-399 .  百度学术
百度学术
22. 李亿芳,金文. 认知图式教育模式对胰腺癌化疗患者认知水平及自我效能的影响. 当代护士(上旬刊). 2023(03): 142-145 .  百度学术
百度学术
23. 董军强,解非,张智翔,贾方. 多层螺旋CT与MRI扫描对胰腺癌和胰腺炎的鉴别诊断. 实用临床医药杂志. 2023(09): 8-12 .  百度学术
百度学术
24. 杨宇,雷建灵. 胰腺癌药物治疗的选择. 临床合理用药. 2023(17): 177-181 .  百度学术
百度学术
25. 马超,曲宁,赵婷,王淑萍,郑秋惠,杨金坤. 杨金坤辨治胰腺癌经验. 中国中医药图书情报杂志. 2023(05): 181-183 .  百度学术
百度学术
26. 张若琪,臧晓彤,张铮,缪锐,王菁,黄蓉,张培彤. 张培彤教授从病-证-症模式辨治胰腺癌经验. 中国医药导报. 2023(25): 120-123+142 .  百度学术
百度学术
27. 闫赵斌,李明彦,李振华,聂山文,饶鹏鹏. 超声内镜检查对胰腺癌及其临床分期的诊断价值. 癌症进展. 2023(15): 1684-1686+1701 .  百度学术
百度学术
28. 沈方力,李叶,汪金辰,茅俭英,吴萃,刘世友. 2009—2021年上海市宝山区居民胰腺癌的死亡趋势和减寿分析. 上海预防医学. 2023(09): 889-892 .  百度学术
百度学术
29. 赵宇婷,杨宇晨,蔡智慧. 液体活检技术在胰腺癌早期筛查和诊断中的应用. 国际检验医学杂志. 2023(24): 3054-3058 .  百度学术
百度学术
30. 刘满洲,化祥帆,孙君军. 血清miR-203和miR-221表达在胰腺癌早期诊断中的应用. 肝胆外科杂志. 2023(05): 386-390 .  百度学术
百度学术
31. 张敏,徐杰茹,陈磊,段朝晖,姚承志,让蔚清. 基于GBD数据分析1990—2019年中国胰腺癌发病趋势. 现代预防医学. 2022(07): 1159-1164+1169 .  百度学术
百度学术
32. 白国辉,任静,董玮琪,刘慧敏,王玉莹,刘世宇,李伟,席云峰. 2017年内蒙古肿瘤登记地区胰腺癌发病与死亡现状及2011—2017年趋势分析. 中国肿瘤. 2022(06): 442-449 .  百度学术
百度学术
33. 战楚婷,张境丰,陈梦池,刘江华. 基于网络药理学研究灯盏花素抗胰腺癌的分子机制研究. 岭南急诊医学杂志. 2022(03): 234-238 .  百度学术
百度学术
34. 肖祥,吴宣諭. 胰腺癌“炎-癌”转化关键基因筛选及干预中药的预测分析. 中草药. 2022(15): 4795-4806 .  百度学术
百度学术
35. 乔炜超,田甜,夏青,张妞. 益气活血解毒方对胰腺癌晚期胰腺细胞迁移和侵袭的影响. 中医学报. 2022(09): 1934-1940 .  百度学术
百度学术
36. 周永婕,王正峰,张泽亮,王海平,徐雯,黄瑶,张磊,周文策. 胰腺癌术后复发的相关危险因素分析. 现代肿瘤医学. 2022(18): 3330-3335 .  百度学术
百度学术
37. 蔡仕良,蒲蕊,柳东红,李子帅,周鑫宇,陈宏森,何奕达,曹广文. 筛查在三类恶性肿瘤精准预防策略中的意义. 上海预防医学. 2022(07): 705-711 .  百度学术
百度学术
38. 刘永鹏,张晶晶,任艳,田庆,刘洪庆. 1990—2019年中国胰腺癌疾病负担变化趋势研究. 现代预防医学. 2022(17): 3079-3085+3110 .  百度学术
百度学术
39. 冯程程,许传志,何杰宇,梁雪萌,孙浩,常巍. 1990-2019年中国20~84岁人群胰腺癌发病趋势分析. 中华肿瘤防治杂志. 2022(18): 1323-1329 .  百度学术
百度学术
40. 张村国,朱宏. 多层螺旋CT多期扫描诊断胰腺癌的准确率分析. 大医生. 2022(17): 119-122 .  百度学术
百度学术
41. 王宝泉,张培彤. 张培彤教授治疗胰腺癌术后恢复期临床经验撷英. 中医药导报. 2022(10): 112-114 .  百度学术
百度学术
42. 张莉娜,张霁雯,罗酩,赵苏鸣. 胰腺癌组织CTTN和miR-545-3p表达水平及其与临床病理和预后的相关性研究. 现代检验医学杂志. 2022(06): 99-103+118 .  百度学术
百度学术
43. 郑立春,张晓明,余天颖,李洁,邓小倩,欧阳向柳. ~(18)F-FDG PET/CT、超声造影及联合应用对胰腺良恶性病变的鉴别诊断价值比较. 临床肝胆病杂志. 2022(12): 2774-2779 .  百度学术
百度学术
44. 徐亦君,竺明晨. cfDNA在胰腺癌中的诊断价值. 肿瘤防治研究. 2022(12): 1265-1268 .  本站查看
本站查看
45. 刘建平,杨兴建,胡毅,罗超,刘涛,李懋. 腹腔镜与开腹胰十二指肠切除术治疗胰头癌的疗效对比及术后肿瘤早期复发的随访研究. 现代生物医学进展. 2022(23): 4531-4535 .  百度学术
百度学术
46. 李小梅,黄海力,崔明新,宋昱,邱娇娇,范利. 对老年患者临终前气管插管的回顾性调查. 中华老年多器官疾病杂志. 2022(11): 817-821 .  百度学术
百度学术
其他类型引用(34)




 下载:
下载: