Efficacy and Safety of Vemurafenib in Acral and Mucosal Melanoma Patients with BRAF Gene Mutation
-
摘要:目的
探索维莫非尼在BRAF基因突变的肢端和黏膜型黑色素瘤患者治疗中的有效性和安全性。方法回顾性分析2011年1月—2016年1月在北京大学肿瘤医院确诊为BRAF基因突变的肢端或黏膜型黑色素瘤住院、并接受维莫非尼治疗患者(24例)的临床资料,随访数据截至2017年1月,所有患者均出现死亡终点。利用Fisher确切概率法检验肢端型和黏膜型黑色素瘤患者的基线资料差异,Kaplan-Meier法分析患者的生存期和无进展生存期,Log rank法检验两种类型黑色素瘤患者生存数据的差异。
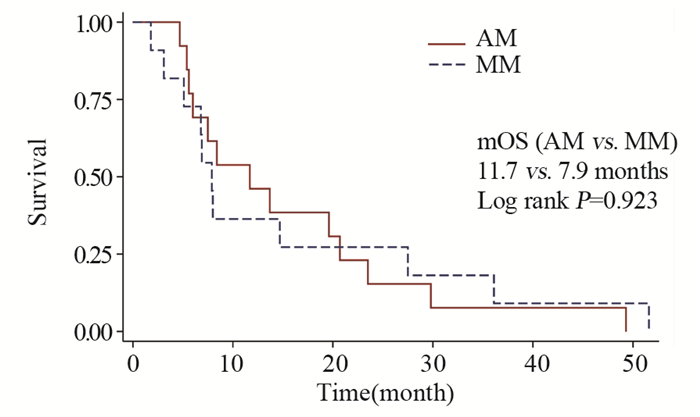
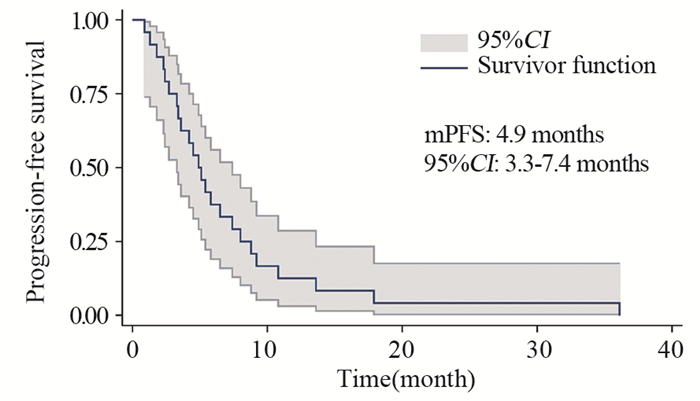
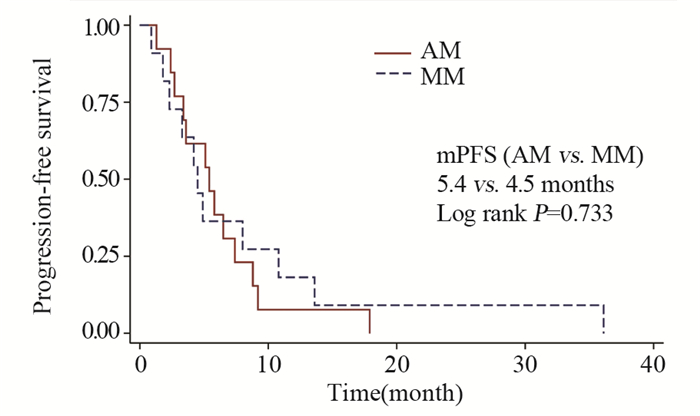
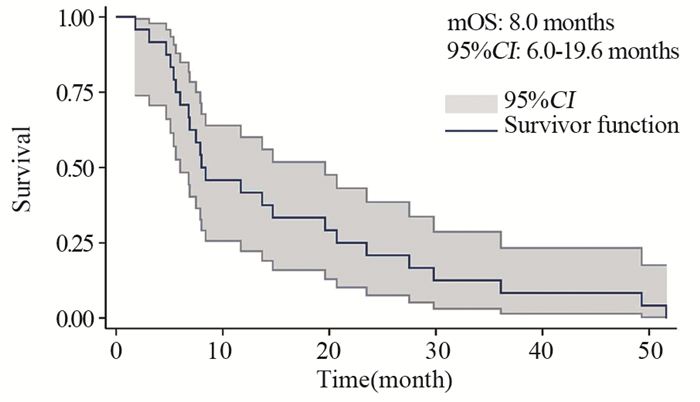
结果基线资料在肢端和黏膜型黑色素瘤患者之间差异无统计学意义。总体的中位生存期和中位无进展生存期分别为8.0月和4.9月。肢端和黏膜型黑色素瘤患者的中位生存期、中位无进展生存期、疾病控制率差异均无统计学意义。维莫非尼不良事件多为3级以下,患者耐受性良好。
结论与皮肤型黑色素瘤相似,维莫非尼在BRAF基因突变的肢端和黏膜型黑色素瘤患者中的有效性和安全性良好。
Abstract:ObjectiveTo explore the efficacy and safety of Vemurafenib in acral and mucosal melanoma patients with BRAF gene mutation.
MethodsWe retrospectively analyzed the clinical data of 24 patients diagnosed as BRAF gene-mutant acral melanoma (AM) or mucosal melanoma (MM) hospitalized in Peking University Cancer Hospital from January 2011 to January 2016. All patients had died by the follow-up cutoff date of January 2017. Fisher exact test was used to detect the difference of baseline characteristics between AM and PM patients. Kaplan-Meier method was used for the estimation of overall survival (OS) and progression-free survival (PFS). We compared patients' survival data between two melanoma types using Log rank test.
ResultsThere was no statistical difference in baseline characteristics between AM and MM patients. Overall median OS and PFS were 8.0 and 4.9 months. No statistical difference was found in median OS, median PFS or disease control rate. The severity of most adverse events was less than grade 3. Vemurafenib was well tolerated during patients' treatment.
ConclusionVemurafenib has acceptable efficacy and safety in BRAF-gene-mutant AM and MM patients, which is similar to that in cutaneous melanoma patients.
-
Key words:
- Acral melanoma /
- Mucosal melanoma /
- Vemurafenib /
- Efficacy /
- Safety
-
-
表 1 AM和MM患者基线信息和最佳疗效
Table 1 Baseline characteristics of AM and MM patients and best response

表 2 肢端和黏膜型黑色素瘤患者维莫非尼不良事件发生情况
Table 2 Adverse events of AM and MM patients treated with vemurafenib

-
[1] Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012[J]. Int J Cancer, 2015, 136(5): E359-86. doi: 10.1002/ijc.29210
[2] Kim KB, Kefford R, Pavlick AC, et al. Phase Ⅱ study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor[J]. J Clin Oncol, 2013, 31(4): 482-9. doi: 10.1200/JCO.2012.43.5966
[3] Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation[J]. N Engl J Med, 2011, 364(26): 2507-16. doi: 10.1056/NEJMoa1103782
[4] Robert C, Long G V, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation[J]. N Engl J Med, 2015, 372(4): 320-30. doi: 10.1056/NEJMoa1412082
[5] Si L, Kong Y, Xu X, et al. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort[J]. Eur J Cancer, 2012, 48(1): 94-100. doi: 10.1016/j.ejca.2011.06.056
[6] Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma[J]. N Engl J Med, 2010, 363(9): 809-19. doi: 10.1056/NEJMoa1002011
[7] Safaee Ardekani G, Jafarnejad SM, Khosravi S, et al. Disease progression and patient survival are significantly influenced by BRAF protein expression in primary melanoma[J]. Br J Dermatol, 2013, 169(2): 320-8. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=1699f7165f0b3f7a59a7356460ee5a4c
[8] Postow MA, Hamid O, Carvajal RD. Mucosal melanoma: pathogenesis, clinical behavior, and management[J]. Curr Oncol Rep, 2012, 14(5): 441-8. doi: 10.1007/s11912-012-0244-x
[9] Spencer KR, Mehnert JM. Mucosal Melanoma: Epidemiology, Biology and Treatment[J]. Cancer Treat Res, 2016, 167: 295-320. doi: 10.1007/978-3-319-22539-5
[10] Butler M, Hamid O, Ribas A, et al. Efficacy of pembrolizumab in patients with advanced mucosal melanoma enrolled in the KEYNOTE-001, 002, and 006 studies[J]. Eur J Cancer, 2017, 72(1): S123. http://cn.bing.com/academic/profile?id=39274f6e8192eb1d7cae3d272c494ed0&encoded=0&v=paper_preview&mkt=zh-cn
[11] Kong Y, Si L, Zhu Y, et al. Large-scale analysis of KIT aberrations in Chinese patients with melanoma[J]. Clin Cancer Res, 2011, 17(7): 1684-91. doi: 10.1158/1078-0432.CCR-10-2346
[12] Bai X, Mao LL, Chi ZH, et al. BRAF inhibitors: efficacious and tolerable in BRAF-mutant acral and mucosal melanoma[J]. Neoplasma, 2017, 64(4): 626-32. doi: 10.4149/neo_2017_419
[13] Chapman PB, Hauschild A, Robert C, et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation[J]. New Engl J Med, 2011, 364(26): 2507-16. doi: 10.1056/NEJMoa1103782
[14] Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib[J]. N Engl J Med, 2012, 366(8): 707-14. doi: 10.1056/NEJMoa1112302
[15] Larkin J, Del Vecchio M, Ascierto PA, et al. Vemurafenib in patients with BRAFV600 mutated metastatic melanoma: an open-label, multicentre, safety study[J]. Lancet Oncol, 2014, 15(4): 436-44. doi: 10.1016/S1470-2045(14)70051-8
[16] Balmelli C, Mark M, Spirig C, et al. Long-term tolerability of the BRAF inhibitor vemurafenib in patients with metastatic melanoma: current study data and real-life observations[J]. memo-Mag Eur Med Oncol, 2014, 7(3): 181-6. doi: 10.1007/s12254-014-0156-6
[17] Blank CU, Larkin J, Arance AM, et al. Open-label, multicentre safety study of vemurafenib in 3219 patients with BRAF(V600) mutation-positive metastatic melanoma: 2-year follow-up data and long-term responders' analysis[J]. Eur J Cancer, 2017, 79: 176-84. doi: 10.1016/j.ejca.2017.04.007
[18] 国家食品药品监督管理总局.药品信息-维莫非尼[EB/OL]. [2017-12-25]. http://app2.sfda.gov.cn/datasearchp/index1.do?tableId=36&tableName=TABLE36&tableView=%BD%F8%BF%DA%D2%A9%C6%B7&Id=16506.China Food and Drug Administration. Medicine information- Vemurafenib film-coated tablets[EB/OL]. [2017-12-25] http://app2.sfda.gov.cn/datasearchp/index1.do?tableId=36&tableName=TABLE36&tableView=%BD%F8%BF%DA%D2%A9%C6%B7&Id=16506.



 下载:
下载: