Effect of Neoadjuvant Chemotherapy Cycles on Pathologic Complete Response Rate Under Different Estrogen Receptor Status of Breast Cancer
-
摘要:目的
分析不同雌激素受体(estrogen receptor, ER)状态下化疗周期数对乳腺癌新辅助化疗病理完全缓解(pathologic complete response, pCR)率的影响。
方法回顾性分析430例乳腺癌新辅助化疗患者的完整病例资料,根据ER状态分为ER+组和ER-组,新辅助化疗周期分为3~4周期和6~8周期。采用χ2检验分析不同ER状态化疗周期数与pCR率相关性,采用多因素Logistic回归分析不同ER状态pCR的独立预测因素。
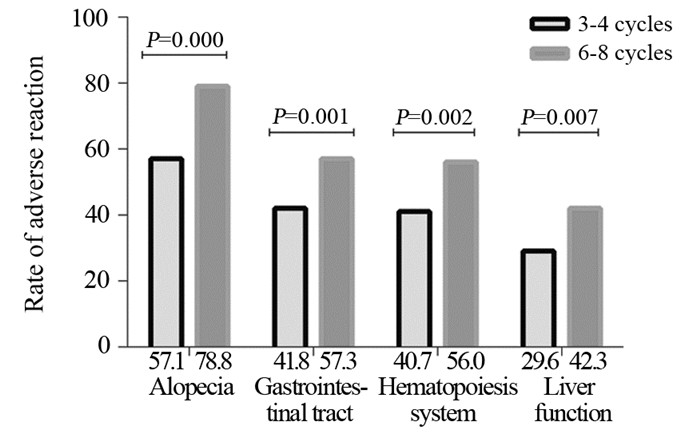
结果430例患者中pCR共103例(24.0%),ER+组6~8周期化疗相比3~4周期化疗使pCR率增加(χ2=7.924, P < =0.005),而ER-组pCR率增加不显著(P < =0.893)。多因素Logistic回归分析提示化疗周期数(P < =0.009)、靶向治疗(P < =0.007)、原发肿瘤大小(P < =0.000)是pCR的独立预测因素。对ER状态分层分析提示,仅在ER+组化疗周期数是pCR的独立预测因素(95%CI: 0.175~0.784, P < =0.009)。
结论6~8周期化疗相比3~4周期化疗可显著提高ER+新辅助化疗患者pCR率,但未显著提高ER-患者的pCR率。
Abstract:ObjectiveTo investigate the correlation between pathologic complete response (pCR) rate and neoadjuvant chemotherapy cycles under different estrogen receptor (ER) status of breast cancer.
MethodsWe retrospectively analyzed the complete clinical data of 430 breast cancer patients in the Tumor Hospital Affiliated to Zhengzhou University from April 1st, 2012 to March 30th, 2016. According to the ER status, the patients were divided into ER+ and ER-groups, and neoadjuvant chemotherapy cycles were divided into 3-4 and 6-8 cycles. The pCR rate of different neoadjuvant chemotherapy cycles were analyzed by χ2 test. The predictors of pCR rate were analyzed using multivariate logistic regression analysis.
ResultsOf 430 patients, the pCR rate were 24.0%(103/430). Compared with 3-4 chemotherapy cycles, the pCR rate was increased in 6-8 cycles in ER+ group, and the difference was statistically significant (χ2=7.924, P < =0.005), while the increased pCR rate was not statistically significant in ER-group (χ2=0.018, P < =0.893). Multivariate logistic regression analysis showed that the chemotherapy cycles (P < =0.009), targeted therapy (P < =0.007), primary tumor size (P < =0.000) were the independent predictors of pCR rate in all patients. The stratified analysis of ER status showed that the chemotherapy cycles was an independent predictor of pCR rate only in ER+ patients (95%CI: 0.175-0.784, P < =0.009).
ConclusionCompared with 3-4 chemotherapy cycles, 6-8 cycles of chemotherapy can significantly improve the pCR rate in ER+ patients, but no significantly increased pCR rate in ER-patients.
-
Key words:
- Breast cancer /
- Neoadjuvant chemotherapy /
- Estrogen receptor /
- Chemotherapy cycles /
- pCR
-
-
表 1 乳腺癌患者的临床病理特征
Table 1 Clinicopathological characteristics of breast cancer patients

表 2 乳腺癌患者不同ER状态化疗周期数和pCR相关性
Table 2 Correlation between pCR and neoadjuvant chemotherapy cycles under different ER status of breast cancer patients

表 3 乳腺癌患者不同ER状态下pCR预测因素的Logistic回归分析
Table 3 Logistic regression analysis of predictors of pCR under different ER status of breast cancer patients

-
[1] Fan L, Strasser-Weippl k, Li JJ, et al. breast cancer in china [J].Lancet Oncol, 2014, 15(7): e279-89. doi: 10.1016/S1470-2045(13)70567-9
[2] 郑莹, 吴春晓, 吴凡, 等.中国女性乳腺癌死亡现况和发展趋势[J].中华预防医学杂志, 2011, 45(2): 150-4. Zheng Y, Wu CX, Wu F, et al. Status and trends of breast cancer mortality in Chinese females[J]. Zhonghua Yu Fang Yi Xue Za Zhi, 2011, 45(2): 150-4.
[3] Mamounas EP. Impact of neoadjuvant chemotherapy on locoregional surgical treatment of breast cancer[J]. Ann Surg Oncol, 2015, 22(5): 1425-33. doi: 10.1245/s10434-015-4406-6
[4] Garg AK, Buchholz TA. Influence of neoadjuvant chemotherapy on radiotherapy for breast cancer[J]. Ann Surg Oncol, 2015, 22(5): 1434-40. doi: 10.1245/s10434-015-4402-x
[5] Hayes DF, Schott AF. Neoadjuvant Chemotherapy: What Are the Benefits for the Patient and for the Investigator?[J]. J Natl Cancer Inst Monogr, 2015, 2015(51): 36-9. doi: 10.1093/jncimonographs/lgv004
[6] Golshan M, Cirrincione CT, Sikov WM, et al. Impact of Neoadjuvant Chemotherapy in Stage Ⅱ-Ⅲ Triple Negative Breast Cancer on Eligibility for Breast-conserving Surgery and Breast Conservation Rates: Surgical Results From CALGB 40603 (Alliance)[J]. Ann Surg, 2015, 262(3):434-9; discussion 438-9. doi: 10.1097/SLA.0000000000001417
[7] Sánchez-Muñoz A, Plata-Fernández YM, Fernández M, et al. The role of immunohistochemistry in breast cancer patients treated with neoadjuvant chemotherapy: an old tool with an enduring prognostic value[J]. Clin Breast Cancer, 2013, 13(2):146-52. doi: 10.1016/j.clbc.2012.11.006
[8] Zambetti M, Mansutti M, Gomez P, et al. Pathological complete response rates following different neoadjuvant chemotherapy regimens for operable breast cancer according to ER status, in two parallel, randomized phase Ⅱ trials with an adaptive study design (ECTO Ⅱ) [J]. Breast Cancer Res Treat, 2012, 132(3): 843-51. doi: 10.1007/s10549-011-1660-6
[9] Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours[J]. Nature, 2012, 490(7418): 61-70. doi: 10.1038/nature11412
[10] Jackisch C, Harbeck N, Huober J, et al. 14th St. Gallen International Breast Cancer Conference 2015: Evidence, Controversies, Consensus-Primary Therapy of Early Breast Cancer: Opinions Expressed by German Experts[J]. Breast Care (Basel), 2015, 10(3): 211-9. doi: 10.1159/000433590
[11] Krishnan Y, Al Awadi S, Sreedharan PS, et al. Analysis of neoadjuvant therapies in breast cancer with respect to pathological complete response, disease-free survival and overall survival: 15 years follow-up data from Kuwait[J]. Asia Pac J Clin Oncol, 2016, 12(1): e30-7. doi: 10.1111/ajco.2016.12.issue-1
[12] Guiu S, Arnould L, Bonnetain F, et al. Pathological response and survival after neoadjuvant therapy for breast cancer: a 30-year study[J]. Breast, 2013, 22(3): 301-8. doi: 10.1016/j.breast.2012.07.012
[13] Swisher SK, Vila J, Tucker SL, et al. Locoregional Control According to Breast Cancer Subtype and Response to Neoadjuvant Chemotherapy in Breast Cancer Patients Undergoing Breast-conserving Therapy[J]. Ann Surg Oncol, 2016, 23(3): 749-56. doi: 10.1245/s10434-015-4921-5
[14] Iwamoto T, Bianchini G, Booser D, et al. Gene pathways associated with prognosis and chemotherapy sensitivity in molecular subtypes of breast cancer[J]. J Natl Cancer Inst, 2011, 103(3): 264-72. doi: 10.1093/jnci/djq524
[15] Yang J, AITahan A, Jones DT, et al. Estrogen receptor-alpha directly regulates the hypoxia-inducible factor 1 pathway associated with antiestrogen response in breast cancer[J]. Proc Natl Acad Sci U S A, 2015, 112(49): 15172-7. doi: 10.1073/pnas.1422015112
[16] Das N, Datta N, Chatterjee U, et al. Estrogen receptor alpha transcriptionally activates casein kinase 2 alpha: A pivotal regulator of promyelocytic leukaemia protein (PML) and AKT in oncogenesis[J]. Cell Signal, 2016, 28(6): 675-87. doi: 10.1016/j.cellsig.2016.03.007
[17] Prossnitz ER, Arterburn JB. International Union of Basic and Clinical Pharmacology. XCVII. G Protein-Coupled Estrogen Receptor and Its Pharmacologic Modulators[J]. Pharmacol Rev, 2015, 67(3): 505-40. doi: 10.1124/pr.114.009712
[18] Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011[J]. Ann Oncol, 2011, 22(8): 1736-47. doi: 10.1093/annonc/mdr304
[19] Schipper RJ, Moossdorff M, Nelemans PJ, et al. A model to predict pathologic complete response of axillary lymph nodes to neoadjuvant chemo (immuno) therapy in patients with clinically node-positive breast cancer[J]. Clin Breast Cancer, 2014, 14(5): 315-22. doi: 10.1016/j.clbc.2013.12.015
[20] Miglietta L, Morabito F, Provinciali N, et al. A prognostic model based on combining estrogen receptor expression and Ki-67 value after neoadjuvant chemotherapy predicts clinical outcome in locally advanced breast cancer: extension and analysis of a previously reported cohort of patients[J]. Eur J Surg Oncol, 2013, 39(10): 1046-52. doi: 10.1016/j.ejso.2013.06.024
[21] Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27[J]. J Clin Oncol, 2003, 21(22): 4165-74. doi: 10.1200/JCO.2003.12.005
[22] Han S, Kim J, Lee J, et al. Comparison of 6 cycles versus 4 cycles of neoadjuvant epirubicin plus docetaxel chemotherapy in stages Ⅱ and Ⅲ breast cancer[J]. Eur J Surg Oncol, 2009, 35(6): 583-7. doi: 10.1016/j.ejso.2009.01.002
[23] Iwata H, Sato N, Masuda N, et al. Docetaxel followed by fluorouracil/epirubicin/cyclophosphamide as neoadjuvant chemotherapy for patients with primary breast cancer[J]. Jpn J Clin Oncol, 2011, 41(7): 867-75. doi: 10.1093/jjco/hyr081
[24] Tanaka S, Iwamoto M, Kimura K, et al. Phase II Study of Neoadjuvant Anthracycline-Based Regimens Combined With Nanoparticle Albumin-Bound Paclitaxel and Trastuzumab for Human Epidermal Growth Factor Receptor 2-Positive Operable Breast Cancer[J]. Clin Breast Cancer, 2015, 15(3): 191-6. doi: 10.1016/j.clbc.2014.12.003
[25] Fei F, Messina C, Slaets L, et al. Tumour size is the only predictive factor of distant recurrence after pathological complete response to neoadjuvant chemotherapy in patients with large operable or locally advanced breast cancers: a sub-study of EORTC 10994/BIG 1-00 phase Ⅲ trial[J]. Eur J Cancer, 2015, 51(3): 301-9. doi: 10.1016/j.ejca.2014.11.023
[26] Moon HG, Im SA, Han W, et al. Estrogen receptor status confers a distinct pattern of response to neoadjuvant chemotherapy: implications for optimal durations of therapy: distinct patterns of response according to ER expression[J]. Breast Cancer Res Treat, 2012, 134(3): 1133-40. doi: 10.1007/s10549-012-2145-y



 下载:
下载:

