Effects of Interferon on PD-1/PD-L1 and Treg Expression in JAK2 V617F-positive Myeloproliferative Neoplasms
-
摘要:目的
探讨干扰素-alpha-2b(IFN-α2b)对JAK2 V617F阳性骨髓增殖性肿瘤(MPN)患者中程序性死亡受体-1(PD-1)、程序性死亡配体-1(PD-L1)及CD4+ CD25+ Foxp3+调节性T细胞(Treg)表达的影响及临床意义。
方法收集JAK2 V617F阳性MPN患者61例,包括初治组41例、IFN-α2b治疗组20例,健康对照组20例。应用荧光定量PCR检测JAK2 V617F/JAK2突变率,流式细胞术检测PD-1、PD-L1、Treg的表达情况。选取15例患者骨髓及外周血标本进行体外细胞培养,应用1×106 U/L IFN-α2b作用48 h后检测PD-1、PD-L1及Treg表达情况。
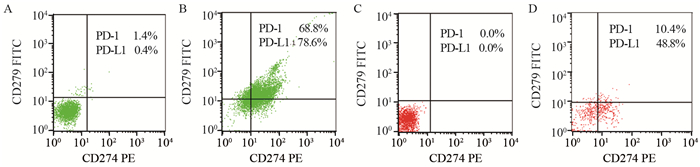
结果初治组的JAK2 V617F、PD-1、PD-L1及Treg表达明显高于IFN-α2b治疗组及对照组(均P < 0.05)。JAK2 V617F突变量≥50%患者骨髓髓系细胞PD-1、PD-L1及外周血Treg细胞均明显高于突变量 < 50%患者(均P < 0.05)。相关性分析结果显示JAK2 V617F突变量与骨髓髓系细胞PD-1、PD-L1和淋巴细胞PD-1呈正相关,与Treg表达无相关性。1×106 U/L IFN-α2b作用48 h后能够体外抑制MPN原代细胞PD-1、PD-L1及Treg的表达(P < 0.05)。
结论PD-1、PD-L1及Treg共同参与了MPN的发病过程,干扰素能够不同程度抑制MPN JAK2 V617F、PD-1、PD-L1及Treg表达,进而抑制MPN的进展。
Abstract:ObjectiveTo explore the effect of interferon-alpha-2b(IFN-alpha 2b) on the expression of programmed death receptor-1(PD-1), programmed death ligand-1(PD-L1) and CD4+ CD25+ Foxp3+ regulatory T cell (Treg) in JAK2 V617F-positive myeloproliferative neoplasms(MPN) and related clinical significance.
MethodsWe collected 61 cases of JAK2 V617F-positive MPN patients, including 41 cases as the newly diagnosed group, 20 cases as the IFN-α2b treatment group and 20 healthy volunteers as control group. JAK2 V617F/JAK2 ratio was detected by fluorescence quantitative polymerase chain reaction (FQ-PCR). The expression levels of PD-1 and PD-L1 in bone marrow and Treg in peripheral blood were detected by flow cytometry. The bone marrow and peripheral blood samples from 15 patients were selected and treated with 1×106U/L IFN-α2b for 48h; and then the expression levels of PD-1, PD-L1 and Treg were detected.
ResultsThe expression levels of JAK2 V617F, PD-1, PD-L1 and Treg in the newly diagnosed group were significantly higher than those in IFN-α2b treatment group and control group(P < 0.05). The expression levels of PD-1, PD-L1 and Treg in the patients with JAK2 V617F/JAK2 ratio≥50% were significantly higher than those with mutation rate < 50%(P < 0.05). JAK2 V617F burden was positively correlated with PD-1, PD-L1 in bone marrow and PD-1 in lymphocyte, while not correlated with Treg. The expression of PD-1, PD-L1 and Treg in primary MPN cells were inhibited by IFN-α2b after 48 hours(P < 0.05).
ConclusionPD-1, PD-L1 and Treg participate in the pathogenesis of MPN together. Interferon could inhibit the progress of MPN via inhibiting the expression of JAK2 V617F, PD-1, PD-L1 and Treg.
-
Key words:
- PD-1 /
- PD-L-1 /
- Treg /
- Myeloproliferative neoplasms(MPN) /
- Interferon
-
作者贡献张丽军:负责文章的数据统计、撰写、修改工作; 齐峰、成志勇、梁文同:导师组成员,负责课题的构思及设计工作; 张朝、吴士杰:负责流式细胞学实验部分; 郭艳涛、孙丽娜、彭占仙:负责收集病例及数据采集工作
-
表 1 JAK2 V617F阳性骨髓增殖性肿瘤不同组JAK2 V617F、PD-1、PD-L1及Treg表达量的关系(x±s)
Table 1 Expression of JAK2 V617F, PD-1, PD-L1 and Treg in different groups of JAK2 V617F-positive myeloproliferative neoplasms (x±s)

表 2 JAK2 V617F突变量与PD-1、PD-L1及Treg的表达的关系(x±s)
Table 2 Relationship between JAK2 V617F and expression of PD-1, PD-L1 and Treg (x±s)

表 3 IFN-α2b作用前后髓系细胞PD-1、PD-L1,淋巴细胞PD-1及Treg表达量的比较(x±s)
Table 3 Comparison of PD-1, PD-L1 expressions in myeloid cells, PD-1 expression in lymphocyte, Treg expression before and after IFN-α2b treatment (x±s)

-
[1] Zhang ZR, Duan YC. Interferon apha 2b for treating patients with JAK2V617F positive polycythemia vera and essential thrombocytosis[J]. Asian Pac J Cancer Prey, 2014, 15(4): 1681-4. doi: 10.7314/APJCP.2014.15.4.1681
[2] 付建珠, 徐倩, 赵亚玲, 等.干扰素抑制JAK2 V617F阳性骨髓增殖性肿瘤血管新生的机制[J].中华医学杂志, 2015, 95(46): 3727-32. doi: 10.3760/cma.j.issn.0376-2491.2015.46.002 Fu JZ, Xu Q, Zhao YL, et al. Anti-angiogenic effect of interferon on JAK2V617F positive myeloproliferative neoplasms and its anti-angiogenic mechanisms[J]. Zhonghua Yi Xue Za Zhi, 2015, 95(46): 3727-32. doi: 10.3760/cma.j.issn.0376-2491.2015.46.002
[3] 赵亚玲, 张丽军, 付建珠, 等. IFN-α2b对JAK2 V617F突变的骨髓增殖性肿瘤COX-2表达及血管新生的影响[J].四川大学学报(医学版), 2016, 47(4): 473-8. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QKC20162016080300049204 Zhao YL, Zhang LJ, Fu JZ, et al. Effect of IFN-α2b on COX-2 and Angiogenesis in JAK2V617F Mutation Myeloproliferative Neoplasms[J]. Sichuan Da Xue Xue Bao(Yi Xue Ban), 2016, 47(4): 473-8. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QKC20162016080300049204
[4] Quintás-Cardama A, Abdel-wahab O, Manshouri T, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon a-2a[J]. Blood, 2013, 122(6): 893-901. doi: 10.1182/blood-2012-07-442012
[5] Sakai S, Kawamura I, Okazaki T, et al. PD-1/PD-L1 pathway imparis T(h)1 immune response in the late stage of infection with Mycobacterium bovis bacillus Calmette-Gué rin[J]. Int Immounol, 2010, 22(12): 915-25. doi: 10.1093/intimm/dxq446
[6] Dieterich LC, Ikenberg K, Cetintas T, et al. Tumor-Associated Lymphatic Vessels Upregulate PDL1 to Inhibit T-Cell Activation[J]. Front Immunol, 2017, 8: 66.
[7] Gravelle P, Burroni B, Péricart S, et al. Mechanisms of PD-1/PD-L1 expression and prognostic relevance in non-Hodgkin lymphoma: a summary of immunohistochemical studies[J]. Oncotarget, 2017, 8(27): 44960-75.
[8] Lu LF, Rudensky A. Molecular orchestration of differentiation and function of regulatory T cells[J]. Genes Dev, 2009, 23(11): 1270-82. doi: 10.1101/gad.1791009
[9] Yi T, Li X, Yao S, et al. Host APCs augment in vivo expansion of donor natural regulatory T cells via B7H1/B7.1 in allogeneic recipients[J]. J Immunol, 2011, 186(5): 2739-49. doi: 10.4049/jimmunol.1002939
[10] Jelinek T, Mihalyova J, Kascak M, et al. PD-1/PD-L1 inhibitors in haematological malignancies: update 2017[J]. Immunology, 2017, 152(3): 357-71. doi: 10.1111/imm.2017.152.issue-3
[11] DoiT, Ishikawa T, Okayama T, et al. The JAK/STAT pathway is involved in the upregulation of PD-L1 expression in pancreatic cancer cell lines[J]. Oncol Rep, 2017, 37(3): 1545-54. doi: 10.3892/or.2017.5399
[12] 查莉, 于姣姣, 许斌.天然免疫检查点CD47-SIRPα在恶性肿瘤中的研究进展[J].肿瘤防治研究, 2018, 45(8): 604-8. doi: 10.3971/j.issn.1000-8578.2018.18.0217 ZHA L, YU JJ, XU B. Research Progress of CD47-SIRPα Signaling Axis as An Innate Immune Checkpoint in Cancer[J]. Zhong Liu Fang Zhi Yan Jiu, 2018, 45(8): 604-8. doi: 10.3971/j.issn.1000-8578.2018.18.0217
[13] 成志勇, 黄月华, 梁文同, 等.骨髓增殖性肿瘤中JAK2 V617F突变与Ⅰ型细胞因子受体相关性研究[J].中国全科医学, 2012, 15(9): 1019-22. doi: 10.3969/j.issn.1007-9572.2012.09.022 Cheng ZY, Hang YH, Liang WT, et al. Correlation between JAK2V617F Mutation and the Expression of Type Ⅰ Cytokine Receptors in Myeloproliferative Neoplasms[J]. Zhongguo Quan Ke Yi Xue, 2012, 15(9): 1019-22. doi: 10.3969/j.issn.1007-9572.2012.09.022
[14] Li N, Wang J, Zhang N, et al. Cross-talk between TNF-α and IFN-γ signaling in induction of B7-H1 expression in hepatocellular carcinoma cells[J]. Cancer Immunol Immunother, 2018, 67(2): 271-83. doi: 10.1007/s00262-017-2086-8
[15] Lai Q, Wang H, Li A, et al. Decitibine improve the efficiency of anti-PD-1 therapy via activating the response to IFN/PD-L1 signal of lung cancer cells[J]. Oncogene, 2018, 37(17): 2302-12. doi: 10.1038/s41388-018-0125-3
[16] Downs-Canner S, Berkey S, Delgoffe GM, et al. Suppressive IL-17A+Foxp3+ and ex-Th17 IL-17Aneg Foxp3+ Treg cells are a source of tumour-associated Treg cells[J]. Nat Commun, 2017, 8: 14649. doi: 10.1038/ncomms14649
[17] Zhou Q, Munger ME, Highfill SL, et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia[J]. Blood, 2010, 116(14): 2484-93. doi: 10.1182/blood-2010-03-275446
[18] Mehrotra S, Sharma B, Joshi S, et al. Essential role for the Mnk pathway in the inhibitory effects of type Ⅰ interferons on myeloproliferative neoplasm(MPN) precursors[J]. J Biol Chem, 2013, 288(33): 23814-22. doi: 10.1074/jbc.M113.476192



 下载:
下载:

